PFE / BNTX, MRNA and AZN / Oxford are the 3 most sensitive names.
The design of dosage and PFE/BNTX provides you with benefits over mr.A.
AZN remains a black horse, however, old American studios have different protocols and an American is just getting started.
BioNTech SE (BNTX) and Pfizer (PFE), Moderna (MRNA), AstraZeneca (AZN) and Oxford are the race leaders for the approval of a COVID-19 vaccine in the US.But it’s not the first time I think PFE/BNTX will probably be the first to report the outcome of a trial in the US.UU.de 30,000 patients, the minimum trial duration that the FDA has requested to ensure emergency use authorization.However, a review of forward-looking progress is warranted.
PFE / BNTX BNT162b2, an RNA vaccine that encodes “one or more SARS-CoV-2 optimized peak glycoproteins”, decided from BNT162b1 based on the effects of a previous clinical examination.BNT162b1 will still be tested through BNTX and Fosun Pharma in China.The BNT162b2 phase 2/3 PFE/BNTX test consists of two doses, separated by 21 days.PFE and BNTX announced the start of this study on July 27, comprises up to 30,000 participants and includes spaces where “significant transmission of SARS-CoV-2” is expected.Research in these regions is attractive because a higher rate of occasions (the proportion of those expanding COVID-19) will help find a difference between placebo and BNT162b2.
On August 20, 3 weeks and 3 days after the start, PFE revealed that it had enrolled 11,000 participants in the 30,000-participant exam.A moment dose was already in progress (which makes sense as the dose at the moment reaches 21 days).Although recruitment was already rapid at the time of the August 20 press release, more test sites were opened in Turkey, Germany and South Africa.On August 26, an PFE scientist reported that the exam now had more than 50% of participants (>15,000 participants).PFE/BNTX plans to submit it for approval in October if the Phase 2/3 exam is successful.PFE begins examining instances of COVID-19 to compare and vaccinate only seven days after the dose at the moment.
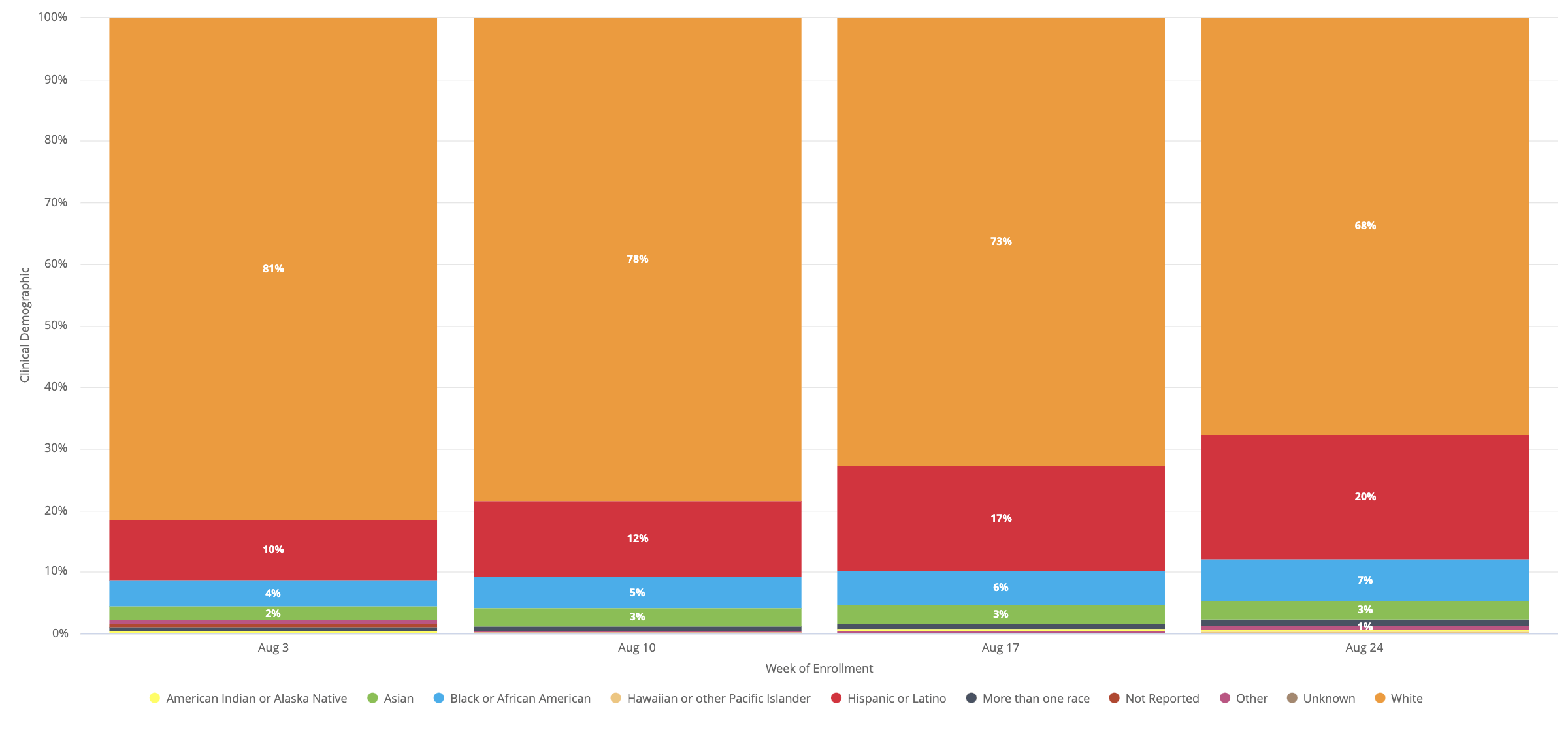
While Blacks and Latinos make up more than 50% of Covid-19 instances nationwide, they only represent about 15% of participants in the country’s first large-scale clinical trial to verify a coronavirus vaccine. , based on knowledge received through CNN. of a government official.
CNN article through Elizabeth Cohen, August 18, 2020.
With the August 21 update, the mnR received an update with some main points on minorities in the mNA test.
As of Friday, August 21, black or African-American, Latino, native, and Alaska native participants accounted for approximately 18% of all participants in the phase study.
MRNA COVE website.
The CNN article discussed the option that COVE examines can also simply slow down recruitment to ensure that more minorities are recruited, so mRNE may fall into the PFE/BNTX.
A similar update occurred on August 28 when the company noted that the number of listings is now 17,458.It appears that the mNR has managed to expand the proportion of minorities in the trial.