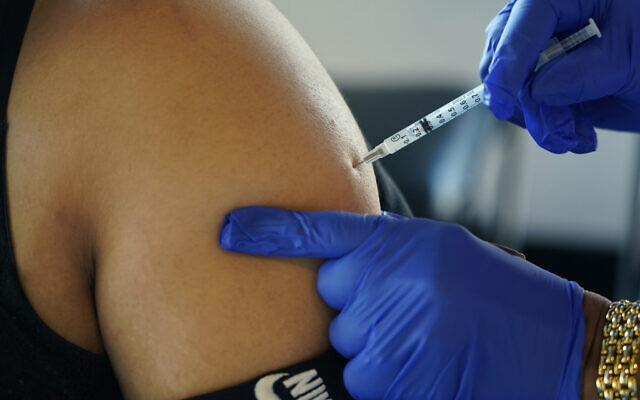
The United States on Wednesday legalized updated COVID-19 boosters for children as young as 5, aiming to expand coverage ahead of an expected winter wave.
Last month, modified boosters were released for Americans 12 and older, doses modified to target today’s maximum non-unusual and contagious Omicron. While there hasn’t been much rush, federal fitness officials are urging others to seek additional coverage before holiday gatherings.
Now, the Food and Drug Administration has given school-age children the green light to also get updated booster doses, one made through Pfizer for children ages five to 11 and an edition of rival Moderna for younger children as 6.
The Centers for Disease Control and Prevention, which recommends the use of vaccines, has approved it.
Americans may be tired of repeated calls to push against COVID-19, but experts say the updated plans have an advantage: They involve partly the recipe that targeted the original coronavirus strain and partly coverage against the dominant versions BA. 4 and BA. 5 Omicron.
These combined, or “bivalent,” boosters are designed to expand immune defenses so that other people are more resistant to serious illness, whether they encounter a relative of Omicron in the coming months, or another mutant that looks more like the original virus.
“We need to have the most productive of both worlds,” Dr. Bill Gruber, a pediatrician at Pfizer, told The Associated Press. He hopes the updated clichés “will reinvigorate interest in protecting young people during the winter. “
The updated reminders are “extremely important” for keeping young people healthy and in school, said Dr. Jason Newland, a pediatric infectious disease specialist at the University of Washington in St. Louis.
Parents want to know “that there are no protective considerations with bivalent, Moderna or Pfizer vaccines,” Newland added.
Only other people who got their first shots, with one of the versions of the original formula, are eligible for an updated booster. That means about three-quarters of Americans over the age of 12 are eligible. Last weekend, only at least thirteen million others had gained an updated reminder, White House COVID-19 coordinator Dr. Ashish Jha estimated Tuesday.
To the chagrin of pediatricians, vaccinating children for the first time has been more difficult. Less than a third of children aged five to 11 gained their two number one doses and would be eligible for the new booster.
The organization of this age will get the children’s doses of the new booster targeting Omicron, and they can get it at least two months after their last dose, either their number one vaccination series or a previous booster, the FDA said.
“Vaccination remains the most effective measure to save you from the serious consequences of COVID-19,” Dr. Peter Marks, the FDA’s director of vaccines, said in a statement.
While young people have a tendency to get sick less severely than adults, “as other waves of COVID-19 occurred, more young people became ill from the disease and were hospitalized,” Marks said.
For the updated recall by Pfizer and its partner BioNTech, children ages five to 11 would receive one-third of the dose already being received by anyone 12 and older. Pfizer said it could ship up to 6 million doses to children within a week of authorization, in addition to ongoing shipments of adult doses.
Until now, the updated Moderna retreat was only allowed for adults. The FDA has just extended this bivalent dose for adults to children 12 to 17 years old and has as legal part the dose for children from 6 to 11 years old.
As for even younger toddlers, the first vaccines weren’t opened for under-5 organizing until mid-June, and it will be several more months before regulators make a decision on whether they’ll also want a booster using the date prescription.
What exactly does an updated COVID-19 booster shot offer?It’s hard to tell. Pfizer and Moderna begin studies in young children.
But the FDA allowed COVID-19 booster changes without requiring human verification effects, just as it approves annual changes to flu vaccines. That’s partly because either corporation had in the past studied experimental designs modified to target earlier variants of COVID-19, adding an earlier edition of Omicron, and found that they were safely activating antivirus antibodies.
“It’s obviously a better vaccine, an improvement than we had before,” Jha said earlier this week.
Jha suggested adults catch up on vaccinations in October, as if they were getting a flu shot, or at least well before holiday gatherings with a circle of high-risk family and friends. People who have recently had COVID-19 still want the booster can still wait about 3 months, he added.
Do you depend on The Times of Israel for accurate and insightful facts about Israel and the Jewish world?If so, sign up for The Times of Israel community. For as little as $6 a month, you:
That’s why we introduced The Times of Israel ten years ago: to provide discerning readers like you with the must-have politics of Israel and the Jewish world.
So now we have a request. Unlike other media outlets, we have not set up a paywall. But because the journalism we do is expensive, we invite readers for whom The Times of Israel has become vital to help our paintings join the Times of Israel community.
For just $6 a month, you can help our quality journalism while enjoying The Times of Israel AD-FREE, and access exclusive content only for members of The Times of Israel community.
Thank you, David Horovitz, founding editor of The Times of Israel.