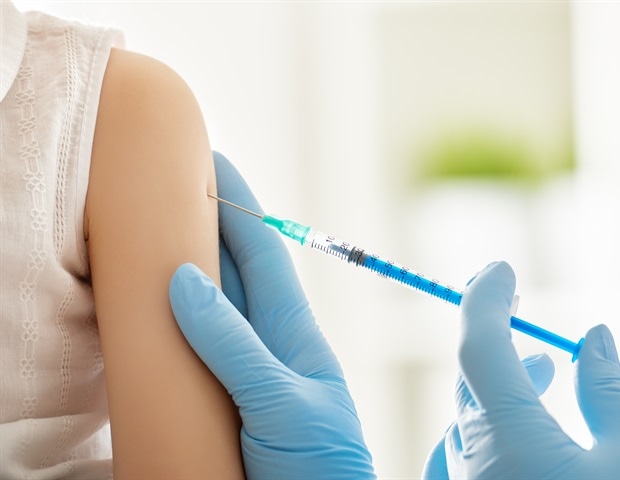
University hospitals today announced their goal of participating as a study in the Global Phase 2/3 study sponsored by Pfizer Inc. and BioNTech SE of an experimental vaccine, BNT162b2, versus SARS-CoV-2. The UH exam is one of 120 clinical researches worldwide that will bring together up to 30,000 participants. This vaccine is one of the most complex applicants for the BNT162 program being evaluated in the United States and Germany and recently won the Fast Track designation of the U.S. Food and Drug Administration (FDA).
This is encouraging news for Ohio. We were among the first in the country to verify Remdesivir, the promising drug in the COVID-19 remedy, which allowed our network to gain advantages from our participation in the clinical trials program. And now, once again, we are bringing to our network some other forward-looking defense mechanism in our fight against coronavirus: a vaccine candidate. The trial was approved by the FDA and our Institutional Review Committee. We anticipate the shipment of the experimental vaccine and the launch of the test next week. “
The trial will be conducted at UH Cleveland Medical Center with Robert Salata, MD, chair of the Department of Medicine at UH Cleveland Medical Center, program director at UH Roe Green Center for Travel Medicine – Global Health and professor of medicine, epidemiology and international. Health at Case Western Reserve University, as principal investigator. In addition, Elie Saade, MD, Director of Infection Control at UH and Scott Fulton, MD and George Yendewa, MD, Assistant Professors of Medicine at UH Cleveland Medical Center, will serve as co-researchers.
“The desire for an effective vaccine is essential in combating the COVID-19 pandemic,” Dr. Salata said. “Lately there is no cure for the new highly contagious coronavirus that causes COVID-19 and our most productive attack plan is to locate a vaccine that can help prevent others from getting it in the first place. The trials we are preparing to conduct are significant because if it is effective and the vaccine receives regulatory approval, Pfizer and BioNTech expect to be able to manufacture up to one hundred million doses by the end of 2020.”
The Phase 2/3 trial is designed as a 1:1 experimental vaccine candidate for a placebo, randomized, blind to discharge the safety, immune reaction and efficacy knowledge needed for regulatory review of this unique vaccine progression approach, which uses mR packaging. with coronavirus genes to generate the complex protein that is intended to generate protective antibodies. The goal is to recruit non-pregnant adults between the ages of 18 and 85.
Due to the disproportionate onset of COVID-19 in other people of color, as well as the severity of the disease and the higher mortality rate, UH plans a forged representation of this population organization in its study.
The main evaluation criterion for the trial will be the prevention of COVID-19 in others who were not inflamed with SARS-CoV-2 prior to vaccination and prevention of COVID-19, regardless of whether participants have already inflamed with SARS. -CoV-2. Secondary criteria come with the prevention of severe COVID-19 in these groups.
“The goal, as always, is to ensure the ultimate state-of-the-art remedies for our UH patients and the communities we serve,” Dr. Simon concluded.
Those interested in participating in the UH exam should call 612-524-9091 for more information.
University of Cleveland Hospital Medical Center
News-Medical.net – An AZoNetwork site
Ownership and operation through AZoNetwork, © 2000-2020