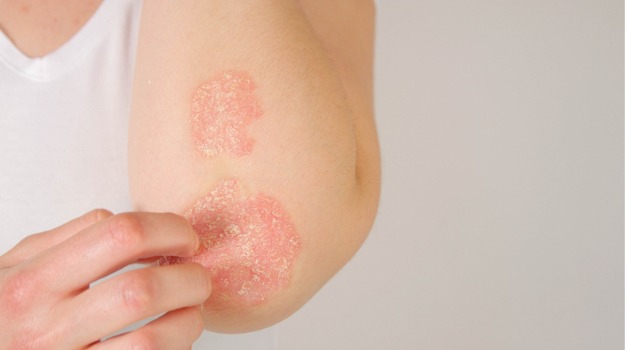
Bimzelx is a humanized IgG1 monoclonal antibody that acts through IL-17A and IL-17F, two central actors in the inflammatory cascade. Bimzelx is the first FDA-approved plaque psoriasis drug that inhibits any of the cytokines, according to UCB’s announcement.
“We know that perfectly transparent skin is valued by other people with psoriasis,” Emmanuel Caeymaex, executive vice president of immunology at UCB, said in a statement, adding that the approval of Bimzelx may increase the popularity of care for the disease.
“Now that BIMZELX is approved for psoriasis, we will continue to temporarily introduce programs for other indications in the U. S. “We are not going to be able to do anything about the U. S. ,” Caeymaex said.
Bimzelx is administered with a prefilled syringe or a 160 mg/mL single-dose auto-injector, and its approval covers adult patients who are also applicants for regimen treatment or phototherapy. Its label does not have a precautionary box but includes precautions opposed to suicidal ideation and behavior, infections, inflammatory bowel disease, tuberculosis, and liver biochemistry abnormalities.
UCB supported their application to the FDA with knowledge of 3 Phase III studies. The first, called BE VIVID, Bimzelx with placebo and J’s Stelara (ustekinumab)
Taken together, the data from those three studies showed that Bimzelx can induce higher levels of skin transparency than placebo, Stelara, and Humira. In particular, more than 80% of the treated patients achieved transparent or almost transparent skin, which is explained as at least 90%. improvement in psoriasis surface domain and severity index (PASI).
Complete dermal clearance, explained as a 100 percent improvement in PASI, was observed in approximately 60% of patients treated with Bimzelx.
Wednesday’s approval is the culmination of a long and somewhat complicated regulatory road for Bimzelx. In October 2021, UCB reported that the FDA had to delay its verdict on the antibody biologics license application because the regulator was unable to conduct on-site inspections of services due to coronavirus-related restrictions.
In May 2022, the delay ended with a full reaction letter, in which the FDA pointed to “pre-approval inspection observations” that the company had to respond to before the application could be reconsidered.
The approval of Bimzelx is UCB’s second regulatory victory this week. The biopharmaceutical company got another green light from the FDA on Tuesday for its subcutaneous C5 inhibitor supplement, zilucoplan, now billed as Zilbrysq, for the treatment of generalized myasthenia gravis.
Tristan Manalac is an independent scientist based in Metro Manila, Philippines. He can be reached at tristan@tristanmanalac. com or tristan. manalac@biospace. com.
Back to news