University of California, Davis Press Release:
August 21, 2020
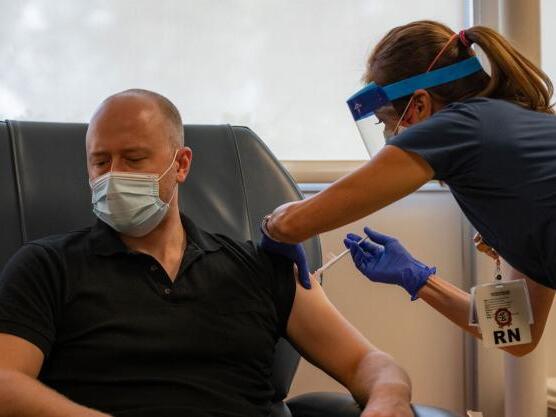
On Thursday, August 20, UC Davis Health patients were the first in Sacramento to obtain a candidate vaccine for COVID-19, a component of a primary clinical trial involving 30,000 participants worldwide.
The other six people who won the injections are part of a complex exam that will recruit approximately 200 participants as part of UC Davis Health’s clinical trial program.
“I am very happy to have the opportunity to fight this pandemic globally,” said one of the first people vaccinated: Lucas Solano, 27, who enrolled in the trial a few days ago. “It is exciting to be one of the other people who would necessarily take the initiative and it would be useful if this vaccine were approved and distributed around the world.
The trial conducted through Pfizer Inc. and BioNTech is ongoing and still accepts participants. Find out what to register for and how to register.
The study trial, known as the Phase 2/3 study, aims at the efficacy and appearance effects of a modified messenger RNA candidate (modRNA) of unmarried nucleoaspect for the BNT162 mRNA-based vaccination program of pharmaceutical companies.
The candidate vaccine has undergone rigorous evaluation in the United States and Germany and has already shown significant results, according to Pfizer.
UC Davis Health, which includes the only college sacramento medical center, is one of 120 trial sites. Pharmaceutical giant Pfizer, which has partnered with Germany’s smallest BioNTech, has decided to examine vaccine sites known for its expertise in world-class studies, infrastructure and concentrations close to known and expected positive cases of COVID-19.
UC Davis has been an active response to coronavirus disease since UC Davis Medical Center providers in February diagnosed and treated the first obvious case of COVID-19 purchased through the network in the United States.
“This is a historic day for UC Davis Medical School,” Dean Allison Brashear said Thursday. “It’s a game changer for our ability to start fighting the pandemic.”
In the randomized trial, some of the participants will get the vaccine and part of the placebo. Patients will know what they are receiving, and neither will the maximum researchers. The test will measure the safety, immune reaction and efficacy knowledge needed for regulatory review.
The vaccine is one of many vaccines developed around the world, at a record rate, with the goal of preventing the spread of the virus that has killed more than 170,000 people in the United States, adding up to about 12,000 in California.
“Accelerating a vaccine like this, given the climate with the virus, is incredibly huge,” said Chris Kain, nursing manager and associate director of the Clinical Research Center at the Center for Clinical and Translational Sciences. Kain coordinated with several departments before the first participants won their photos.
“It’s amazing to see all the groups run together,” he said.
UC Davis Health serves a geographic domain populated by races and ethnicities, expanding the possibilities of identifying clinical trial applicants from more varied backgrounds compared to other communities in the United States. Latinos and blacks have been disproportionately affected by the virus and are encouraged to enroll in the study. Healthcare personnel and those working in environments with a large number of customers, such as grocery stores, are encouraged to participate.
The main purpose of the trial is to save COVID-19 in others who were not inflamed with SARS-CoV-2 prior to vaccination and to save COVID-19, whether participants have already been inflamed with SARS-CoV- or not. 2. A secondary objective is to avoid coVID-19 bass in these groups.
If the vaccine candidate’s good luck continues, Pfizer and his European spouse BioNTech have said they are on track to request a regulatory review starting in October. If regulatory approval or approval is obtained, corporations plan to supply up to one hundred million doses by the end of 2020 and approximately 1.3 billion doses by the end of 2021.
See above coverage: UC Davis Health to recruit participants for the COVID-19 vaccine primary clinical trial
Follow Dateline UC Davis on Twitter.
This press release produced through the University of California, Davis. The reviews expressed here are those of the author.
Patch is a network news area. Please keep your answers clear, kind and objective. Read our network rules here
Loading…