Ending the covid pandemic would likely require a vaccine that protects against any new strains. The researchers probably would have found a strategy that will work.
Long before Alexander Cohen, or anyone else, heard about the alpha, delta, or omicron variants of covid-19, he and his graduate counselor Pamela Bjorkman were conducting studies that could soon allow for a single vaccine to defeat the evolving virus, as well as any other variants of covid-19 that may occur in the future.
Before the pandemic, Cohen had been a doctoral student in the Bjorkman Structural Biology Laboratory at the California Institute of Technology, and attempted to design a new type of “universal” flu vaccine. the flu virus that the pathogen would not be able to adjust or disguise even as it evolved.
So in early 2020, when covid-19 hit and he was about to graduate soon, Cohen, Bjorkman, and other members of the lab set out to design a universal covid vaccine, a vaccine that would provide coverage not only against all its variants, but also opposes long-term illnesses caused by entirely new types of coronavirus.
“We’re going to want something like this to combat covid-19 as new variants emerge,” Cohen says. “But beyond that, the prospect of new epidemics and global pandemics caused by other coronaviruses is clear. We want anything that can save the new covid-19 type scenarios from falling again. And we want it as soon as possible.
Scientists and public fitness officials have long complained about a lack of investment, or a sense of urgency, to expand vaccines that would protect us from pandemics in the long run. However, driven by COVID-19, the U. S. National Institutes of Health (N. C. )millions of dollars to study teams for universal coronavirus vaccines.
The stakes may be higher. In January, Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, called the progression of universal coronavirus vaccines “urgently needed,” noting that the emergence of COVID-19 variants in the more than two years portends a long-term threat. Since then, he has argued that even more resources are needed to continue the fight, and has publicly insisted on lawmakers to allocate them.
This new type of biodesigned vaccine may be the answer we desperately want for long-term coronavirus pandemics.
“Scientific evidence and ecological truth that coronaviruses will reappear in the future, which could pose an existential threat,” Fauci wrote in a paper co-authored with two other infectious disease experts for the New England Journal of Medicine.
The key to putting together the challenge, according to teams like Bjorkman’s, possibly lies in our ability to use artificial biology equipment to trick the microscopic weapons of the immune formula, weapons that already exist in the human body. Researchers are locating tactics to overfeed those immune cells to provide remarkably overall coverage against invading microbes. If those approaches are successful, not only can they provide much more effective coverage against covid, but they will also revolutionize the way we create new vaccines for complex viruses in general.
After helping pave the way for the progression of those techniques, Cohen, Bjorkman and their collaborators are now on the verge of achieving their goal of producing a vaccine that largely triggers an immune reaction not only against covid and its variants, but also against it. to a wider variety of coronaviruses.
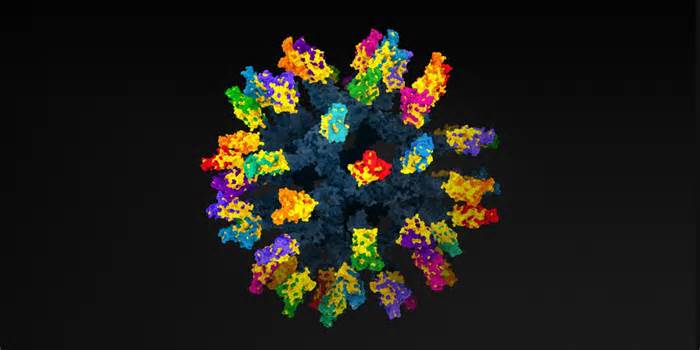
Their vaccine is composed of a round protein core, peppered with a soccer-like tendency with “sharp” protein spikes taken from the surface of 8 types of coronavirus, what scientists call mosaic nanoparticle. Surprisingly, the first effects showed that in a control tube, antibodies produced through this artificial vaccine were able to identify and adhere not only to the 8 coronaviruses represented in the nanoparticle, but also to 4 more coronaviruses that were not used in the vaccine. In March, the organization reported that the vaccine gave the impression to mice and monkeys that had been exposed to a variety of coronaviruses.
The new messenger RNA vaccines to fight the coronavirus are based on generation that could be just medicine. Then: sickle cell anemia and HIV.
In July, they published the effects in Science, appearing that their mosaic nanoparticle vaccine for non-human mice and primates of the delta and beta covid-19 variants, as well as the human viruses that caused the first SARS outbreak in 2003. The effects are perhaps the most promising evidence that this new type of biodesigned vaccine may be the answer we desperately want to avoid coronavirus pandemics in the long term.
The next step is the vaccine in humans. The Coalition for Epidemic Preparedness Innovations will provide up to $30 million to begin human trials. Edinburgh-based biotech company Ingenza will manufacture the drug.
Since this technique is new, the start of the test can take up to two years. But if it succeeds, we may not have to undergo another covid-related lockdown again.
Covid-19, like many viruses, has proven to be a master of disguise. It relies on the most difficult tool in variety of herbs, random mutations, to adjust its shape in a way that, at least in the case of the omicron variant. , allows you to be more cunning than the most ubiquitous tool the framework uses to prevent viruses: antibodies.
Antibodies are Y-shaped proteins that in the blood bind to the surface of express pathogens and wrap them in an immobilizing bear hug until they can be killed. To protect us from anything nature can offer us, the human body is provided with the ability to make a probably infinite variety of antibodies, each with a slightly different shape. The interlocking amino acids that combine to shape the two arms of an antiframe shape distinct shapes designed to interact like Lego blocks in complementary proteins discovered on the surface of a fast-invading pathogen.
The viruses that cause us the most disruption are the ones that can stay one step ahead of the human immune formula (and our maximum productive efforts to stimulate it with the help of vaccines), by converting the shape of its surface proteins fairly quickly, or through the evolution into the bureaucracy that makes it difficult for an antibody to bind to them. The proteins on the surface of HIV, for example, are so far apart that only one arm of the antibody’s Y can bind. Those inflamed with the flu virus mutate and evolve so much into a new bureaucracy that the antibodies we produce no longer interact perfectly and lose the ability to adhere to them. That’s why we want a new vaccine every year to stay.
But what if we could simply identify a way so that the structural integrity of a virus can never mutate or replace, what biologists call a “conserved” feature, and then engineer a microscopic particle to adhere to that shape?
“There are parts of a lot of viruses that don’t change,” Cohen says. “But unfortunately, our bodies do a much poorer job of detecting those preserved sites. It turns out that there is a preference for the reaction of antibodies to recognize highly variable sites. And viruses are smart at converting the portions that the immune formula recognizes to the maximum without problems. “
In 2019, Cohen immersed himself in a task to expand a universal flu vaccine that led the immune formula to target conserved spaces discovered on the surface of maximum flu viruses. It ran with an evolved generation through Mark Howarth, a protein biologist at the University of Oxford: a self-assembled nanoparticle with 60 open dots on its surface, each designed to have qualities similar to those of Velcro. These points are designed to attract and bind with molecules that have a loose lab-designed Velcro patch on their surfaces.
Biologists like Cohen can put those complementary Velcro-like labels on any protein, and that protein will stick to the nanoparticle. In its final form, this particle self-assembles into a bristling design similar to a soccer ball dotted with a mosaic of proteins of other shapes, held in position with the help of a protein superglue.
The technology, which Howarth has made available to research teams around the world, makes it possible to selectively design vaccines. Cohen and his colleagues began experimenting with diversification proteins in the flu virus, measuring the ability of other combinations to save it. new strains of flu infecting mice. He had just finished completing his PhD and was about to start a new series of experiments.
Then came covid-19.
Cohen first learned about the mysterious new virus emanating from Wuhan, China, by browsing his favorite website, an online infectious disease tracker that monitors new outbreaks in humans and animals around the world. When he discovered that his efforts to design a universal flu vaccine were temporarily halted, he immediately advised Bjorkman to launch a task by applying the same technique to covid, seeking to identify the preserved parts of the covid-19 virus, SARS. -CoV-2, which can also be provided in other SARS – such as viruses – and manufacture a vaccine targeting them.
In April 2020, Cohen returned to the lab. To locate the bureaucracy on the viral surface that would likely be preserved, he and Bjorkman relied on a giant framework of clinical literature that characterized and compared the genetic sequences of coronaviruses.
Most coronaviruses, including the one that causes covid-19, consist of a genetic curtain wrapped in a protein and enclosed in a protective membrane similar to a soap bubble, which is difficult to distinguish for the immune formula from the outer membrane surrounding humans. . But there’s an Achilles’ heel: claw-like proteins that protrude across the membrane so that the virus can harbor vulnerable cells long enough to inject its genetic curtain and require the cell’s protein-producing machinery to make copies of itself. These spikes have distinct shapes that, in the case of covid-19, are designed to interact on proteins called ACE2 receptors, which are found on the surface of many human cells.
Spikes are hot targets for antibodies. But they mutate smoothly to shape new forms that allow them to evade detection. Of the 53 new mutations known in the omicron variant, for example, 30 involve the spike protein gene. Thirteen of them shape 3 distinct organizations, two of which modify the tip near its end while the third organization modifies the domain closer to the base. Together, those mutations adjust the shape of the beak enough to allow it to escape antibodies that would bind strongly to other versions of the covid-19 virus.
The key to the universal vaccine is the mosaic nanoparticle with so many other viral fragments clustered in the vicinity of its surface. It is very likely that the B cells of the immune system, which generate express antibodies, will locate and bind to at least some of those preserved pieces of the virus, which remain unchanged in the new variants. Therefore, B cells will produce effective antibodies opposed even to the new variants.
To make their mosaic of nanoparticles, Cohen, Bjorkman and their collaborators chose proteins on the surface of 12 known coronaviruses through other study teams and detailed in the clinical literature. These included the viruses that caused the first SARS outbreak and the one that caused covid-19, but non-human viruses were also discovered in bats in China, Bulgaria and Kenya. As a smart move, they also dropped a coronavirus discovered on a scaly anteater known as a pangolin. All strains had already been genetically sequenced by other teams and 68-95% percent of the same genomic material. Therefore, Cohen and Bjorkman can be sure that at least some parts of each of the distinct spike proteins they chose to place their nanoparticle outdoors would be divided among some of the other viruses.
The key to the universal vaccine is the mosaic nanoparticle with so many other viral fragments clustered in the vicinity of its surface.
Then they did 3 vaccines. One, for comparison, had all 60 positions occupied by remnants of a single strain of SARS-CoV-2, the virus that causes COVID-19. The other two were mosaics, each of which showed a set of protein fragments from eight of the 12 bat, human and pangolin coronavirus strains. The remaining 4 strains have not been vaccinated so that researchers can check if they would vaccinate them anyway.
In studies with mice, all 3 vaccines also connected well with the covid-19 virus. But when Cohen sat down to look at his results, he was surprised at how much more resilient the mosaic nanoparticles were when exposed to other coronavirus strains not represented in the spikes. had been exposed.
The vaccine triggered the production of armies of antibodies to target the portions of the proteins they replaced the least among the other coronavirus strains, the portions, in other words, that are preserved.
In recent months, Bjorkman, Cohen and their collaborators have tested the vaccine on monkeys and rodents. So far, it turns out it’s working. Some of the experiments progressed slowly as they had to be conducted by foreign collaborators in special high-security biosecurity laboratories designed to ensure that highly contagious viruses did not escape. But when the effects nevertheless gave the impression on Science, the paper gained a lot of attention.
Other promising efforts are progressing in parallel. At the University of Washington’s Protein Design Institute, biochemist Neil King custom-designed a bunch of new types of nanoparticles, “sculpting them atom by atom,” he says, so that atoms self-assemble, drawn through the right position through other portions designed to bring complementary geometric and chemical charges. In 2019, King’s collaborator Barney Graham at the NIH was the first to effectively demonstrate that mosaic nanoparticles can be effective against other influenza strains. King, Graham and their collaborators have formed a company to modify and expand the technique and have a nanoparticle influenza vaccine in Phase 1 clinical trials. They are now deploying the new generation in opposition to a variety of other viruses, adding SARS-CoV-2.
Despite recent promising developments, Bjorkman warns that his vaccine probably won’t protect us from all coronaviruses. There are four families of coronaviruses, each different from the other, and some target completely different receptors in human cells. , there are fewer preserved sites in coronavirus families. His lab’s vaccine focuses on a universal vaccine opposed to sarbecovirus, the subfamily comprising sars coronaviruses and SARS-coV-2.
“I’m not sure it’s ever imaginable to make a pan-coronavirus vaccine for singles,” Bjorkman says. “So we’re just looking to make relatively undeniable fruits, which would be a pan-sarbecovirus vaccine. But I think it’s important, because this is the circle of relatives from which many of the last occasions of overflow have occurred.
In addition, studies in the lab of Bjorkman and others open a new frontier in vaccine design that has implications far beyond their efforts. The paintings would possibly adapt to attack coronaviruses in other families, and even other viruses. it will also bring a new era in vaccine progression in which vaccines that oppose a wide diversity of challenging pathogens can be more easily created and customized.
But the regulatory hurdles they have to overcome are significant. A new vaccine produced through a traditional technique would have to demonstrate “protective correlates” with existing vaccines, evidence that the immune formula responds to the vaccine in the same way it does with existing vaccines. But because mosaic nanoparticle vaccines are new, the researchers want to show that the vaccine prevents Americans from getting sick, which takes much longer and requires more money.
Cohen suggests it may take a few years just to begin testing, as the vaccine will have to go through rigorous toxicology testing and meet strict production criteria to pass the regulatory meeting. But with the initial money raised, a well-known manufacturer, and articles in the world’s most productive clinical journal proving their promise, there are still reasons to be optimistic.
With plans to create realistic, potted embryos, Renewal Bio is on an adventure towards the horizon of science and ethics.
A scientist went in search of the genes responsible for cannabinoid hyperemesis syndrome. But a public discussion with a hashish influencer suffering from the disease would likely have derailed his research.
For the first time, a patient from New Zealand underwent a genetic modification to lower his cholesterol. This may just be the beginning of a new era in disease prevention.
A robust molecular language is spoken between you and your brain.