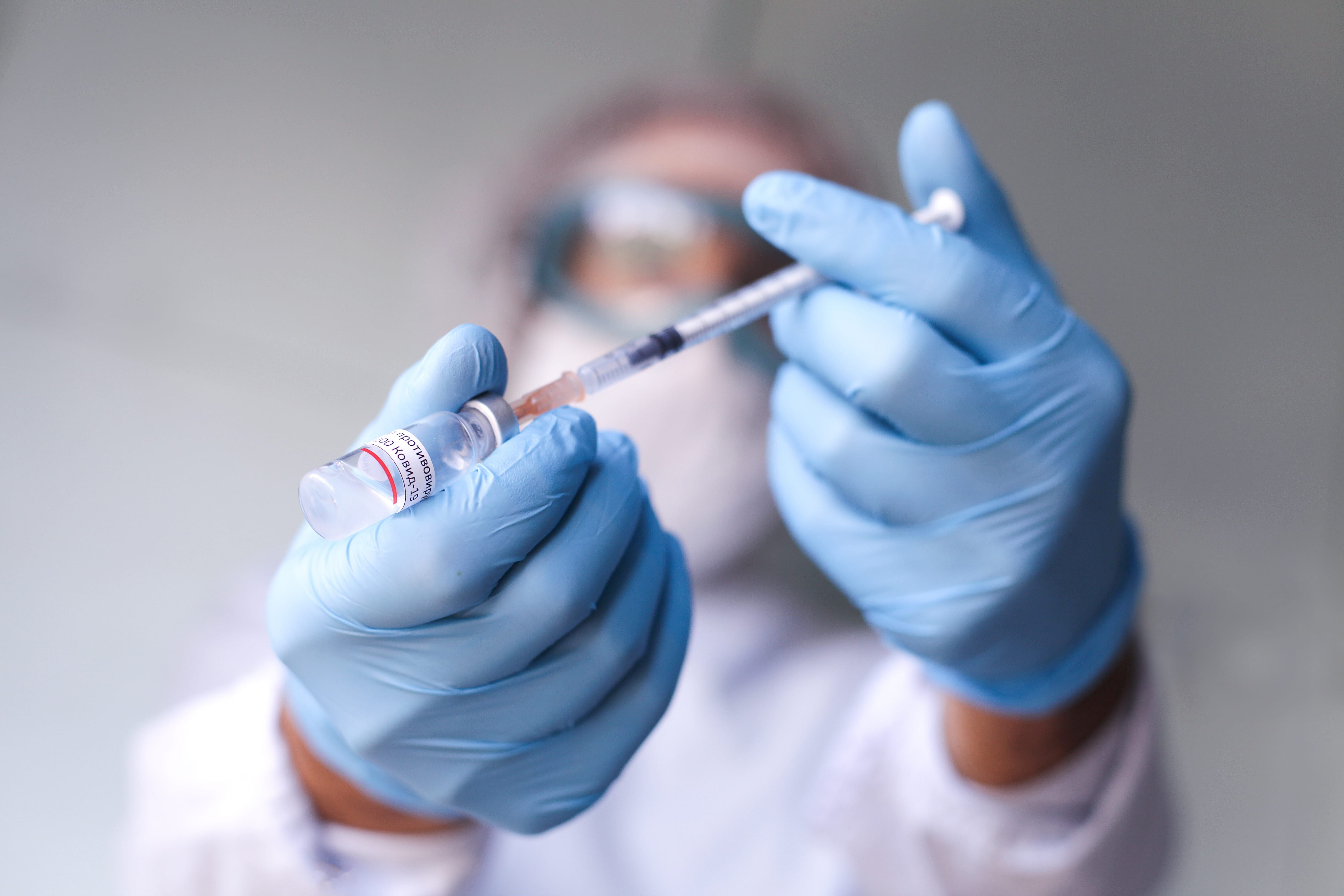
The U.S. government He has promised approximately $5 billion to international coVID-19 vaccine developers to ensure that the first batch of approved vaccines is successful in the Americans, by the end of 2020. However, the Centers for Disease Control and Prevention (CDC) “The most recent estimates recommend that the first batch of vaccines is far from sufficient to protect even the highest-risk population. And it’s not yet transparent to the government who deserves to get vaccinated first.
Depending on the number of vaccine applicants reaching the final line, there may be between 10 and 20 million doses at first, according to a CDC presentation to outdoor vaccination counselors last week, the Wall Street Journal reported. But public fitness officials have said that more than a hundred million Americans, along with fitness staff and other essential staff, are vaccinated before the general public to save lives.
This means that complicated decisions will have to be made as to which of the high-risk organizations will be vaccinated first and how the doses will be distributed to the rest of the population. For example, “this may mean simply prioritizing emergency branch staff in hospitals and extensive care sets over physical care personnel who have less interaction with the sickest patients,” the Journal, which mentioned CDC officials.
Several government agencies and COVID-19 committees are in the process of developing such plans. But we still don’t know who will have the last word.
The resolution will most likely depend on the CDC’s own vaccine committee, the Advisory Committee on Immunization Practices (ACIP), which sometimes makes recommendations on which vaccines the federal government buys and distributes.
However, the CDC and the National Institutes of Health have also asked the National Academies of Science, Engineering and Medicine (NASEM) to form a separate committee to draft a vaccine distribution plan.
President Donald Trump’s own coronavirus task group, Operation Warp Speed, may also have a say in the matter, according to a management officer interviewed through the Journal. The organization is already primarily responsible for the resolution on vaccine developers with whom it contracts the US government’s source of symptoms.
Finally, the Food and Drug Administration (FDA) may also get involved. The firm has a history of adding distribution limits in the drafting of drug approvals. By authorizing the emergency use of Gilead Sciences coronavirus treatment, remdesivir in May, the FDA clarified that the drug will be used for inpatients and not those in home quarantine.
What is at stake is paramount to a successful vaccine on the market on time. “The high probability of vaccines is one of the main reasons why the inventory market has been controlled to succeed at new heights even without a definitive improvement in physical fitness outcomes in the United States,” Goldman Sachs analysts said in a report cited Wednesday through MarketWatch.
If the first COVID-19 vaccine can be widely obtained during the first quarter of 2021, “we could see a really large build-up in GDP compared to a case of ‘non-vaccine,” the bank said, “especially for the United States, which will probably lead the vaccine race and likely see worse effects than in Europe without a vaccine.”
We understand: he likes to be in control of his own experience on the Internet. But advertising revenue helps our journalism. To read our full stories, turn off your ad blocker, we would appreciate it very much.
Below are the steps you can follow to load Observer.com on the target of your browser:
Click the AdBlock button in your browser and do not run on the pages of that domain.
Click the AdBlock Plus button in your browser and Enabled on this site.
Click the AdBlock Plus button in your browser and turn off Observer.com.