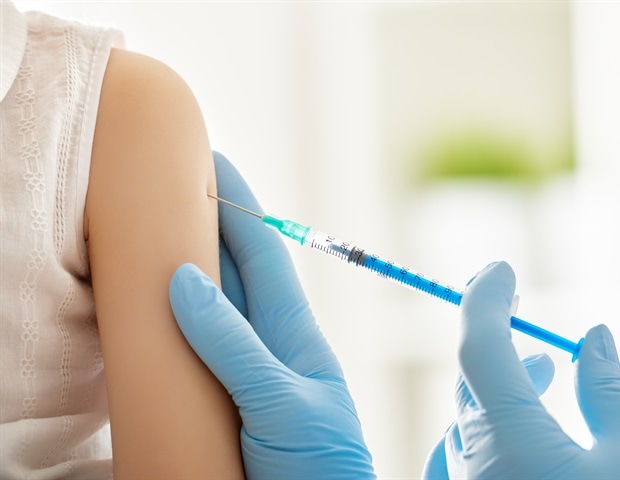
The race to produce vaccines opposed to COVID-19 will have to make sure the deficient are not left behind, Crispin Maslog says.
A crazy race to produce a vaccine opposed to COVID-19 began with the world’s superpowers at the forefront. There are millions of lives and billions of dollars at stake.
Among the pioneers are the United States with its Operation Warp Speed with its futuristic sound; Europe and China also have their own leading candidate vaccines; as the race warms, the least evolved countries in Asia, Africa and South America are cheering and waiting for crumbs, where maximum clinical trials of vaccines will be or are already underway.
Typically, it takes at least 4 years to expand a vaccine before it is marketed, but at the age of COVID-19, fitness experts are positive about a vaccine in a year or less. There’s a sense of urgency and we’re waiting for penetration.
Meanwhile, the most sensitive of the vaccine queue, which is expected to be in condition until the end of the year, are the populations of Western countries, which are, of course, the precedence of their governments that funded studies in First Place.
Deficient Asian countries and the rest of those that come globally sadly have to wait at the end of the line, which is why some of them have agreed to be guinea pigs for vaccine trials in the hope that they will take precedence when vaccines are available. put into service Beggars have no choice.
In mid-August, President Rodrigo Duterte suggested that the Philippines participate in phase 3 trials of the Russian Sputnik vaccine against Saudi Arabia and the United Arab Emirates to enroll the Philippines in clinical trials. The Russian offer of clinical trials in the country can be a catastrophic mistake because Russian assignment is suspicious.
Indonesia has begun a complex human trial of a CoVID-19 vaccine manufactured in China involving up to 1,620 patients. No less than Indonesian President Joko Widodo presented the essay at a rite in Bandung, West Java, in mid-August.
Indonesia’s resolve to be China’s spouse in clinical trials may be a bigger gamble, as China is leading the race to produce a vaccine.
The candidate vaccine produced through Sinovac Biotech is among the few in the world to participate in phase 3 clinical trials, or large-scale human trials, the last step before regulatory approval. Bangladesh too.
Asia is the preferred destination of drug brands for clinical trials for several reasons. These come with medical experience in areas of rapid healing, the availability of giant patient groups, laboratories and infrastructure, comparable quality and lower costs. Another thing is the occurrence and comparables. de of Western diseases.
There is also global data acceptability. Data from clinical trials in Asia are widely accepted through regulatory agencies: the US Food and Drug Administration is widely accepted. (USFDA) and the European Medicines Agency (EMA). In addition, the prices of procedures, diagnostic tests and visits to Asia are 30% to 40%. decline than in the United States and Europe.
As the race heats up, a warning is needed: scientists will have to not sacrifice clinical integrity for policies, but they will have to stick to strict protocols for clinical studies and production. Governments want to put science into politics in the vaccine race.
Safety and efficacy are related to vaccine progression. An error in clinical trials caused by hasty procedures, for example, can result in deaths that will delay studies and progression over several years.
As things are, there is already a “vaccine hesitation” among audiences around the world, especially among the uninformed. Surveys show that U. S. citizens have less self-confidence about vaccine safety.
A May survey through the opinion and knowledge company YouGov found that 55% of U. S. adults said they would get a COVID-19 vaccine. By the end of July, that figure had fallen to 41 in line with the penny, well below 60%. 70 in line with the penny that experts need to achieve “collective immunity. “
There is also significant skepticism about vaccines in other countries, according to a recent Wellcome Trust study. In France, less than part of other people think vaccines are safe. In Ukraine, the most skeptical country in the world, this figure is only 29%. . Do not feed this vaccine with faults.
As superpowers rush to the end line, rhetoric emerges: who gets the vaccines first?Rhetoric because, unless a foreign framework intervenes, we know that the deficient will have the final say. Some countries in Asia and Africa have negotiated agreements, but it is not the maximum in Asia and Africa.
And even for those who have negotiated agreements, there is no guarantee, and if the amount of dose to be received will be sufficient for canopy to the majority of the population.
Unless governments partially or completely subside vaccines, they will be unaffordable to the poor. Early reports imply that Chinese vaccines will charge $145 according to injection into the open market, while those in Oxford, UK, will charge only $4 to $10, as some countries plan to provide loose vaccines, and even pay others to deal with herd immunity, about 70 to 90% of the population.
There is hope on the horizon through COVAX, a consortium of 172 economies being organized and “working with vaccine brands to provide countries around the world with equitable access and effective vaccines once authorized and approved. “
“This is the only global initiative with governments and brands to ensure that COVID-19 vaccines are international for high- and low-income countries,” organizers said in a press release.
In the race to a vaccine, the countries canArray . . . join to participate in an initiative that is based on informed self-interest and also equity, without leaving any country behind. “
This is a welcome progression and we hope it succeeds. Let evolved vaccines and humanity win. No shortcuts, please.
SciDev. Net
News-Medical. net – An AZoNetwork site
Ownership and operation through AZoNetwork, © 2000-2020