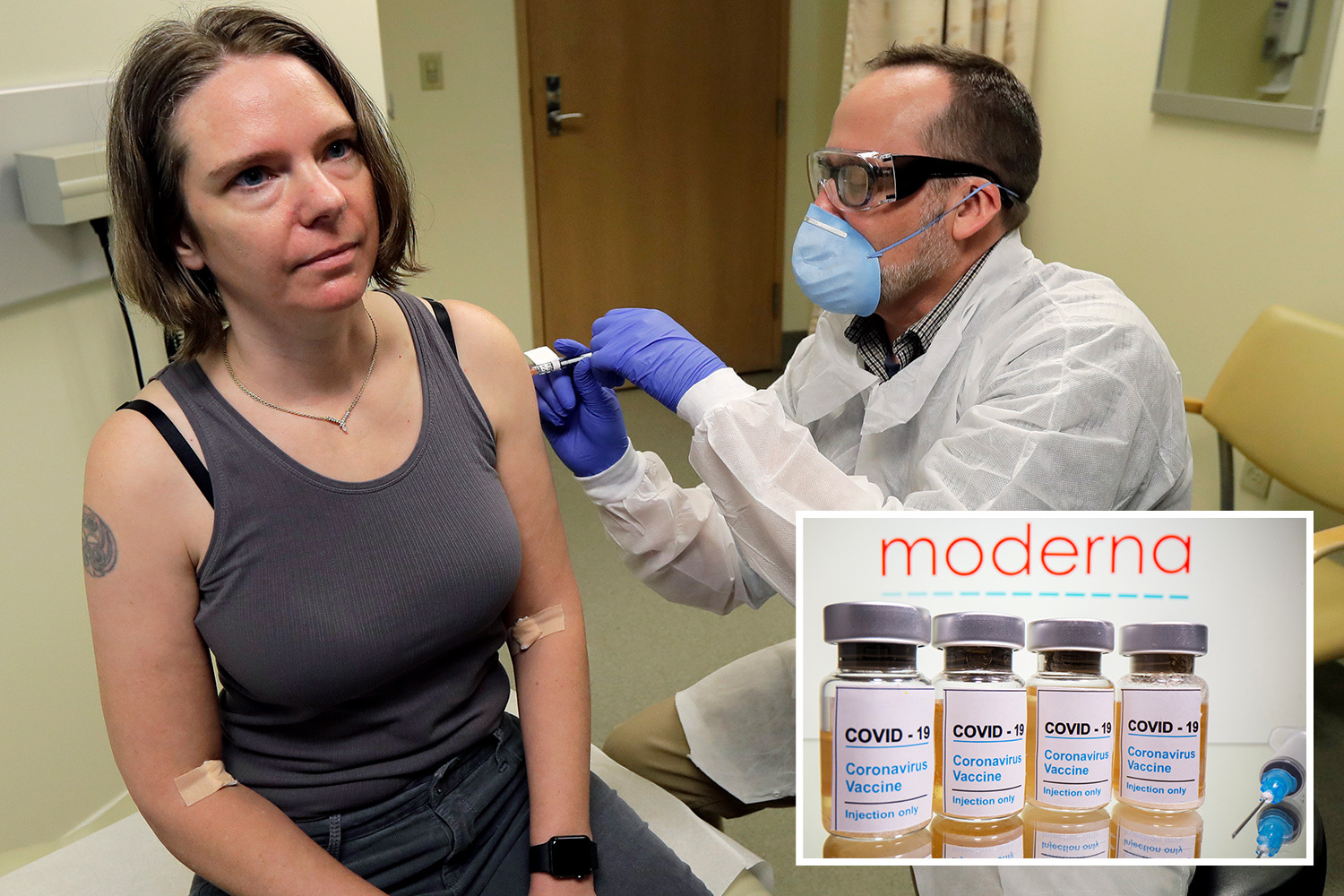
Trials of more than 30,000 people revealed that only five other people who won the Modern vaccine developed Covid, none with severe symptoms.
⚠️ Read our blog about coronavirus for the latest news and updates
By comparison, 90 other people who won a fictitious vaccine became ill, according to the US biotechnology company.
However, the UK has no advance requests and The Sun revealed that ministers are now fighting for safe supplies.
Officials said they were in complex talks to buy millions of injections, which use the same generation of RNA as the Pfizer vaccine.
But it will be the spring of 2021 “at the latest” before the British receive the doses, as the US company expands its european chain of origin, a government spokesman said today.
With the Pfizer vaccine, which has been shown to be 90% effective, it is possible that in the US it is possible that it will be effective in the US. But it’s not the first time Only two injections are allowed for emergency use in December.
The US has an agreement of 1. 16 billion pounds ($1. 5 billion) per hundred million doses, while the EU has an “unsigned” agreement for 160 million doses, he said. -in reported.
Meanwhile, Moderna is in agreements with Japan, Canada, Switzerland, Israel and Qatar to deliver more vaccines.
It is expected to charge $11. 57 ($15. 25) according to the dose, and with two required according to the child, it costs less than Pfizer to 14. 79 ($1950) according to the dose.
However, British scientists praised the ad as “extremely exciting” and say it raises the possibility of several vaccines.
Moderna’s move has also been tested on those at maximum risk of severe Covid disease, adding teams of the elderly and ethnic minorities, offering a touch to other vulnerable people of the fatal virus.
Stéphane Bancel, managing director of Moderna, said: “This is a turning point in the development of our Covid-19 candidate vaccine.
“This positive interim investigation from our Phase 3 exam gave us the first clinical validation that our vaccine can save you from Covid-19 disease, adding serious illnesses. “
This comes a week after Pfizer announced that his Covid puncture is more than 90% effective against the disease.
Britain has booked 40 million doses, and the first 10 million can be administered until Christmas if the vaccine is approved.
But despite promising symptoms, Pfizer’s blow will remain at about -70 degrees C (-94 degrees F), which is much less than what an average freezer can reach.
On the other hand, the Modern Jab can be stored in the refrigerator for a month, which means it would be much more to distribute than Pfizer’s favorite.
Scientists say it stays solid at -20 degrees Celsius for up to six months, in refrigerated situations for up to 30 days and at room temperature for up to 12 hours.
Juan Andrés, Director of Technical Operations and Quality of Moderna, said: “The ability to buy our vaccine for up to 6 months at -20 degrees Celsius, adding up to 30 days in general refrigerator situations after thawing, is a progression and would allow for easier distribution. “
Moderna has also included many seniors and others at high risk in its main clinical trial, which experts say makes the effects more applicable to those who are most vulnerable to Covid.
Early intermediate research included 95 participants who showed cases of Covid-19, of whom 90 had won placebo and five had won the active vaccine.
All 95 instances included 15 older adults, over the age of 65, as 20 non-white people, adding 12 Hispanics or Latinos, 4 African-Americans, 3 Asian-Americans, and a multiracial.
Severe cases of coronavirus, 11 in the first intermediate analysis, were also examined.
All cases occurred in the organization of placebo and none in the organization that won the vaccine, recently known as mRN-1273.
Moderna said his knowledge did not involve any significant security issues.
However, the effectiveness of 94. 5% of this research may decrease as the additional effects of the clinical trial are announced.
Dr. Stephen Hoge, president of Moderna, said he “smiled from ear to ear” upon learning of the effectiveness of the coronavirus vaccine by the US company.
He told BBC News: “When we got the news from the knowledge and safety board, I admit I broke the character and smiled from ear to ear for a minute.
“Since I didn’t expect it, I don’t think any of us were hopeful that the vaccine would be 94% effective in preventing Covid-19 disease, in fact it was an amazing achievement.
“But the time that may be even more exciting is that it is one hundred percent effective, apparently, to save him severe Covid-19 disease.
This means that I think we have the team to nevertheless defeat this virus.
“There were 11 cases of serious illness, all 11 were on placebo, none of those vaccinated had severe Covid-19.
“And this mixture of facts means that the vaccine is actually a wonderful tool to prevent the pandemic and prevent the worst diseases that other people face.
“When you mix it with last week’s news about the Pfizer vaccine, you now have two vaccines that are above 90% effective.
“It means that I think we have the equipment to nevertheless defeat this virus and I think it’s probably the most productive news of the day for all of us, is that there are answers in our hands and that we want to take the other people who can use them. “
Professor Peter Openshaw of Imperial College London said: “This news from Moderna is incredibly exciting and greatly reinforces optimism about opting for the right vaccines in the coming months.
He said: “First we heard the 90% power of Pfizer and BioNTech, then the Russians said 92% and now Modern 94. 5%.
“This most recent press release is in a study of 30,000 American adults, many of them elderly and high-risk.
“This gives us confidence that the effects are applicable to those who are at maximum risk of Covid-19 who want the vaccines to the fullest. “
Professor Openshaw added that garage temperatures would make management much easier than the Pfizer vaccine.
He added: “This is a strong vote of confidence in RNA vaccine technology, which turns out to be safe, appropriate and effective in studies involving 70,000 people. “
“These effects set the tone for the development of other vaccines. “
The experimental medicine professor also said it’s “pretty good” news in terms of side effects, with only a small percentage of symptoms reporting.
“The first dose caused injection pain in about 3% of people,” he said.
“The dose at the moment was related to widespread brief symptoms in approximately 10% of other people with fatigue, muscle pain and flu-like symptoms.
“This implies that they obtained the approximately correct dose with appropriate adverse events.
“These effects are what we would expect with a vaccine that works and induces an immune response. “
As vaccines take years to succeed at this stage, other experts also welcomed the announcement of today’s progress.
Professor Trudie Lang, who runs the Global Health Network at Oxford University, news of the effectiveness of the modern “remarkable” vaccine.
Speaking to BBC News, he said he had worked on vaccines against malaria, tuberculosis and Ebola, but “we’ve never noticed anything like this before. “
Commenting on the previous announcement on the efficacy of the Pfizer vaccine, Professor Lang said: “To have knowledge of two other vaccines, the same generation in two absolutely different trials, but that the same effects appear is exciting. “
When asked how the vaccine progress had been achieved, he said, “The explanation for why we are in such a scenario with Covid is that we have just had this massive collaboration and global focus. “
He explained that clinical collaboration and lessons learned from paintings on an Ebola vaccine had been incorporated into coronavirus paints.
This includes 40 million doses of the promising Pfizer injection, which turned out to be 90% last week.
These are the vaccines that the government ordered in advance:
Oxford / AstraZeneca: One hundred million doses Weakened virus that causes colds in chimpanzees, has been shown to generate a strong immune reaction opposite to Covid-19.
It has been genetically modified, so it is highly unlikely to expand in humans, doing so for children, the elderly and others with pre-existing diseases.
Currently, in Phase 3 trials in the United Kingdom, the United States, South Africa, Japan, Brazil and Kenya, more than 50,000 patients evaluated have won the vaccine. Early reviews showed it’s safe.
An Australian company has already produced millions of vials in the hope that the tests will succeed.
Novavax: 60 million doses
It contains a purified part of the virus that causes Covid-19. When administered, it frames it as “strange” and generates a protective immune response.
It has been shown to generate more antibodies than in patients recovering from severe Covid-19 infections.
Currently in Phase 3 trials in the United Kingdom and the United States.
GSK / Sanofi: 60 million doses
It uses protein as one of Sanofi’s seasonal influenza vaccines, combined with a recall.
In phase 1 clinical trials, the first effects were positive.
Valneva: 60 million doses An inactivated total virus vaccine designed to induce the framework to create higher grades of Covid-19 antibodies.
The government has invested in the Valneva production plant in Livingston, Scotland, to set up a vaccine plant in the UK.
Currently under initial research, trials should begin in December.
Pfizer / BioNTech: millions of doses
Prevents Covid-19 infection through the virus’s “advanced protein,” turning it off before it causes damage.
Tested in 40,000 patients, it is currently undergoing phase 3 trials, however, the first intermediate research has shown that it has 90% efficacy.
Janssen: 30 million doses
It uses a modified bloodless virus to act as a Trojan horse that can deploy the “advanced protein” of the Covid-19 virus in human cells, which causes the framework to produce antibodies.
Phase 3 trials in 60,000 patients were recently discontinued after an unexplained disease in a volunteer. Since then, testing has resumed.
350 million doses in total
Professor Lang warned that he would have a “portfolio” of candidate vaccines in development.
“We have those two messenger RNA vaccines now and there are other technologies, hopefully.
“And it would be a position to have, you know, possibly another 3 or 4 vaccines coming in the coming weeks and months, and possibly even perform a little other tasks, but in fact secure international supply. “
He added that “we just have to wait and see” what knowledge of Pfizer and Modern vaccines has shown.
She added: “We still don’t know what kind of coverage these vaccines will give, whether they will save you the disease and prevent transmission or either, and some of the vaccines that arrive later may work slightly differently.
“So, until we have a full understanding of this, which we might not do in the coming months, then the fitness government can make a resolution on which vaccine to use the right maximum in other situations. “
Professor Stephen Evans, London School of Hygiene
“This is the first study to report serious cases and, in addition to uncertainty, the discovery of the absence of severe cases with the vaccine and 11 cases with placebo is a very solid proof that the vaccine prevents serious and benign diseases.
“The trials included a wide variety of other people with diseases and minority teams, as well as a significant number of older patients.
“We will want much more knowledge and a full report or publication to see if the benefits gained are consistent in all groups, especially the elderly, but it is encouraging progress. “
The 100th FTSE has climbed to its point since early June in reaction to the news.
Number 10 said the UK had been executing agreements with vaccine developers that offer “different types of vaccines, can get an early source in the UK, and have put complex production source chains on position. “
Last October, Moderna announced that the UK Vaccine Regulatory Agency (MHRA), which approves vaccines, had introduced the procedure for continuous review of its vaccine.
Dr. Charlie Weller, Wellcome’s head of vaccines, said: “The hopes of ending this pandemic are vaccines, remedies and effective testing.
“It is incredibly promising that the vaccines we urgently want are now on the horizon.
“Having multiple vaccine applicants with intermediate effects that exceed our expectations is phenomenal and bears witness to this year’s global study effort.
A government spokesman said: “Modern news seems clever and represents a vital step towards the location of an effective vaccine opposed to Covid-19.
“As a component of the ongoing paintings of the Vaccine Working Group, the Government is conducting complex discussions with Moderna to ensure that the UK has its vaccine as a component of the UK’s broader portfolio.
“Modern is recently expanding its chain of European origin, which means that these doses would be obtained in the UK until the spring of 2021 at the latest.
“To date, the UK government has immediately insured 350 million doses of vaccines through agreements with six independent vaccine developers. This includes 40 million doses of the Pfizer/BioNTech vaccine, which is based on the same platform as the Moderna vaccine and, if approved through the drug regulator, is expected to begin administration from December 2020. »
Meanwhile, the UK will now be the first country to conduct end-stage trials for a Covid vaccine developed through the pharmaceutical company Janssen.
It is the third coronavirus vaccine tested in the British, after Oxford and Novavax, and will involve another 6,000 people across the country.
Ministers have earned up to 30 million doses, if successful, with expected effects starting in mid-2021.
Intermediate research from Phase One and Two trials indicated that it induces a “robust immune response” and is well tolerated.
And British drug giant Glaxo-SmithKline revealed that he had already manufactured “millions of doses” of his dose of Covid.
Roger Connor, its global vaccine president, said the company had begun mass production and was preparing to launch the newest trials.
It’s security approval in the first part of next year.
Professor Ugur Sahin, a scientist from Pfizer jab, said yesterday: “If all goes well, we will start administering the vaccine by the end of this year, we will start next year. “
“Our purpose is to deliver more than three hundred million doses of vaccines until April next year, which can allow us to start having an effect now. “
Side effects are accompanied by mild injection pain and fever for a few days.
© News Group Newspapers Limited in England No. 679215 Headquarters: 1 London Bridge Street, London, SE1 9GF. “The Sun”, “Sun”, “Sun Online” are registered trademarks or industrial names of News Group Newspapers Limited. This service is provided under the popular terms and situations of News Group Newspapers ‘Limited, in accordance with our privacy and cookie policy. For information about a hardware replica license, our distribution site. Check out our online press kit. For any additional requests, please contact us. To view all the contents of The Sun, use the site map. Sun’s online page is regulated by the Independent Press Standards Organization (IPSO)
Our hounds aspire to precision, but we make mistakes. To learn more about our claims policy and to register a claim, click here.