ATLANTA – A possible COVID-19 vaccine that Atlanta doctors helped verify has the largest in the world.
The Emory University component of the first clinical trial of the vaccine evolved through the National Institutes of Health and Modern Inc.
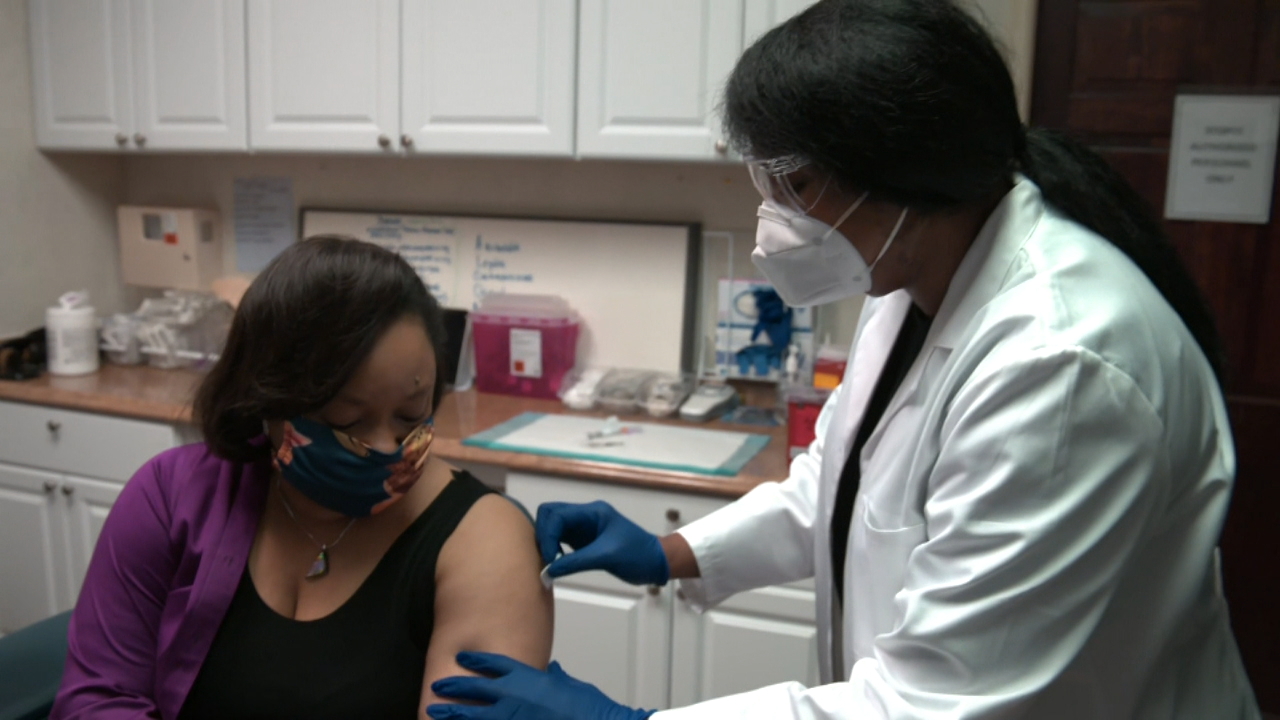
As of Monday, the exam began with the first of 30,000 volunteers who are expected to help verify vaccines. He is one of many applicants in the final stretch of the global vaccine race.
[The ongoing COVID-19 assay in Emory shows effects in opposite combat to the virus, moves to the next phase]
There is still no guarantee that the experimental vaccine will protect.
The test: Volunteers won’t know if they get the genuine photo or a fake version. After two doses, scientists will largely monitor which organization reports maximum infections while painting daily, especially in spaces where the virus is still spreading unchecked.
“Unfortunately for the United States of America, we have a lot of infections right now” to get this answer, Dr. Anthony Fauci of niH told The Associated Press.
Modern said vaccination took place in Savannah, Georgia, the first site to begin among more than seven dozen checkpoints across the country.
Channel 2 host Jorge Estévez spoke to a boy before this month who has already volunteered for the trial.
Sean Doyle, a Ph.D. and a medical student at Emory University volunteered for Ebola trials several years ago, so when asked to volunteer for the first phase of the Trial of Modern’s COVID-19 vaccine, he hesitated.
“I was actually nervous, ” said Dolyle. “With the new drug remedy or the new vaccine, you never know exactly what will happen when it is obtained. But, to be honest, I was very excited to participate.
Several other vaccines manufactured through China and the British University of Oxford earlier this month began smaller final phase tests in Brazil and other heavily affected countries.
But the U.S. They are not easy to test their own vaccines that can be used in the country and have set the bar high: month through autumn, the government-funded COVID-19 prevention network will launch a new test on a leading candidate – with 30,000 newly recruited volunteers.
Mass studies don’t just try to check if vaccines are working, they’re needed to check the protection of every possible vaccine. And following the same review regulations will allow scientists to eventually compare all plans.
Governments around the world are looking to buy millions of doses of these lead candidates, so if regulators approve one or more vaccines, vaccines can start right away. But the first doses obtained will be rationed, possibly reserved for those most exposed to the virus.
“We are optimistic, cautiously optimistic” that the vaccine will work and that “by the end of the year” there will be knowledge to produce it, Dr. Stephen Hoge, president of Moderna, founded in Massachusetts, told a subcommittee in the House of Representatives in the past. Week.
[CONNECTION: FULL LIST: Which cities require face masks?]
Meanwhile, Haller, the volunteer vaccinated in March, wears a mask in public and takes the same precautions away with everyone, hoping that one of the vaccines will occur in the pipeline.
“I don’t know what the chances are that this is precisely the right vaccine. But thank God there are many others fighting this right now,” he said.
The Associated Press data used in this report
© 2020 Cox Media Group