Advertising
Supported by
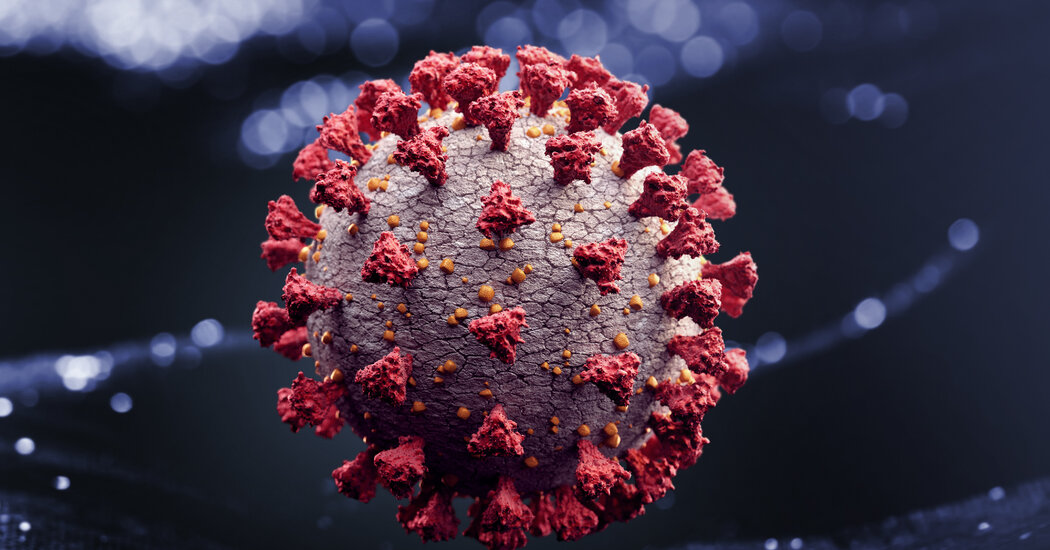
SARS-CoV-2 has been slowly evolving modestly, without further danger.
By Edward Holmes
Dr. Holmes is an evolutionary virologist.
SYDNEY, Australia – Read recent headlines about patients who have recovered from Covid-19 to later re-inflame with SARS-CoV-2, allegedly through another “strain” of the virus.
At the end of August, data on the first case of SARS-CoV-2 reinfection “documented” or “confirmed” to the world: a Hong Kong boy, diagnosed in March, had contracted “a new virus “circulating in Western Europe this summer. The next day, it was learned that two other people in Europe also gave the impression that they had been reinfected.
After that, those were stories about the first American case of its kind, involving a Nevada patient who reportedly suffered worse symptoms at the time.
All this communication about sarS-CoV-2’s newest and most virulent bureaucracy unnecessarily creates concern and confusion.
Let’s take a look at the evidence and science.
On the one hand, remote cases of reinfection also occur with other viruses, which is not necessarily alarming. Reinfection only tells us how the human immune formula works, but at first glance it is not proof that a virus has mutated in a way that makes it more dangerous.
On the other hand, viruses mutate, and most of those adjustments are bad for the virus or even fatal, according to some studies (a minority of mutations are neutral and only a small minority is beneficial). The word “mutation” would possibly seem disturbing, but it is a banal fact of viral life and its implications are sometimes not destructive to humans.
And yes, SARS-CoV-2 is changing. So what?
The genuine question is: was it more virulent or infectious than it was when it was first detected in Wuhan, central China, in December?The evidence suggests no.
Like viruses that transmit flu or measles to us, SARS-CoV-2 has a genetic code composed of RNA or ribonucleic acid, but RNA is highly mutable, and as SARS-CoV-2 infects us through our body’s cells to reflect and reflect once again, each and every time its genome is copied , an error may slide.
Most mutations are lost quickly, either by possibility or because they damage some of the virus’s main functions. Only a small proportion ends up spreading widely or in a lasting way. The mutation may be the fuel of evolution, but, especially for an RNA virus, it is also the quo.
RNA viruses tend to evolve about a million times faster than human genes, however, if SARS-CoV-2 stands out among them, it is to evolve more slowly than many: about five times slower than influenza viruses, for example.
According to Nextstrain, an open source mapping that tracks pathogens in real time and other sources, SARS-CoV-2 accumulates on average about two mutations consistent with the month, meaning that the circulating virus bureaucracy is just another 15 mutations from the first edition attributed to the Wuhan epidemic.
This is a small number since the SARS-CoV-2 genome is approximately 30,000 nucleotides in length and also means that existing versions of the virus are approximately 99. 95% compared to the original Wuhan. is slowly evolving.
(Therefore, talking about SARS-CoV-2 that has become so many other “strains” is misleading. Scientists tend to reserve the word for versions of a virus that differ biologically in primary forms. The other SARS-CoV -2 bureaucracy is very similar; “variants”)
The slow rate of coronavirus mutation is good news for us: a virus that has evolved faster is more likely to outperigh all vaccines or drugs developed to counteract it.
That said, have even small mutations altered SARS-CoV-2 so particularly?
For example, is it deadliest?
To my knowledge, there is no evidence to date that SARS-CoV-2 is more virulent or deadly, let alone.
For example, a recent preprinted article (still peer-reviewed) through Erik Volz of the Faculty of Medicine at Imperial College London and many colleagues from other establishments, adding members of the Covid-19 Genomics UK Consortium, which analyzed 25,000 sequences of the total SARS-CoV-2 genome collected in the UK revealed that a specific mutation of the virus called D614G , had higher mortality in patients.
What about infectiousness?
Much debate has been debated about the D614G mutation, which affects the so-called peak protein of the virus, which has made SARS-CoV-2 more infectious.
The complex protein is on the surface of the coronavirus, and this is because it is the component of the virus that adheres to host cells. “D614G” is a shortcut to replace in position 614 of the complex protein, from an ascomponent acid (D) to an amino acid glycine (G). (Technical documentation refers to “D614” as the above configuration and “G614” as the last).
The D614G mutation, which probably gave the first impression in China, gave the impression that the outbreak in northern Italy was becoming increasingly common in February. The G614 form of the virus has spread internationally and has become the dominant variant.
The D614G mutation appears to have increased coronavirus infection, at least in laboratory-grown cells, according to a recent article through PC biologist Bette Korber and others published in the journal Cell.
Apparently, based on one component of this and other studies, the country fitness government has stated that the G614 form of coronavirus may be only 10 times more infectious than the edition first detected in Wuhan.
But as some epidemiologists have warned, it is difficult, if not reckless, to extrapolate laboratory effects to how the virus actually spreads in a genuine population.
I don’t think the evolution of SARS-CoV-2 is causing the virus to spread continuously. The coronavirus is still smart to spread because most of us are still sensitive to it; we are not immune and can still locate new guests to infect them easily.
In the same Cell factor that korber’s article published, viral epidemiologist Nathan Grubaugh and his colleagues argued that “the accumulation in G614 frequency can be explained simply through the possibility and epidemiology of the pandemic.
I agree.
In other words: the next time you compare other epidemics and start wondering or worrying about diversifications, first assume that those diversifications are similar to situations on the ground, than anything about the virus itself, like a new mutation.
Take, for example, the wave of SARS-CoV-2 infections that has been affecting Australia since June. Although there has been a primary outbreak in Victoria (with a peak of approximately 700 cases consistent with the day), the outbreak in The State of New South Wales has been lower so far (with a daily number of about 10), but either was caused by the same variant of the coronavirus, which has the D614G mutation.
The exact reasons for these differences are still under investigation, however, it can be said that, as the outbreak hit Victoria first, the NSW government had more time to prepare.
Mortality rates also differ from position to position and, in some cases, the virus may appear to kill more people, but again, these diversifications say less about the virus than on differences in how the disease is treated or in places where the virus has spread mainly among vulnerable populations, such as others in nursing homes.
In addition, even if the D614G mutation increases the infectiousness of the virus in humans, this fact does not have a major involvement in our customers to develop an effective vaccine. The mutation affects the complex protein, but not the component of it that the neutralizing antibodies of the human immune formula targets when the framework defends against infection.
Viruses mutate constantly; SARS-CoV-2 is no different. And we continue to monitor when and how, and with what effects, it evolves.
Whether SARS-CoV-2 is more infectious or deadly is a vital issue, especially since it does not appear that the virus will be eliminated in the short term. It is most likely endemic in humans, as everyday as influenza.
For now, however, SARS-CoV-2 is necessarily the same virus that gave the impression in December. Of course, it has mutated, but not, so far, in a way that replaces the way scientists think about how to deal with it. – and not in a way that worries you.
Edward Holmes is an evolutionary virologist at the University of Sydney.
The Times has pledged to publish one of the letters to the editor. We would like to know what you think of this article or one of our articles. Here are some tips. And here’s our email: lettres@nytimes. com.
Follow the New York Times Review segment on Facebook, Twitter (@NYTopinion) and Instagram.
advertising