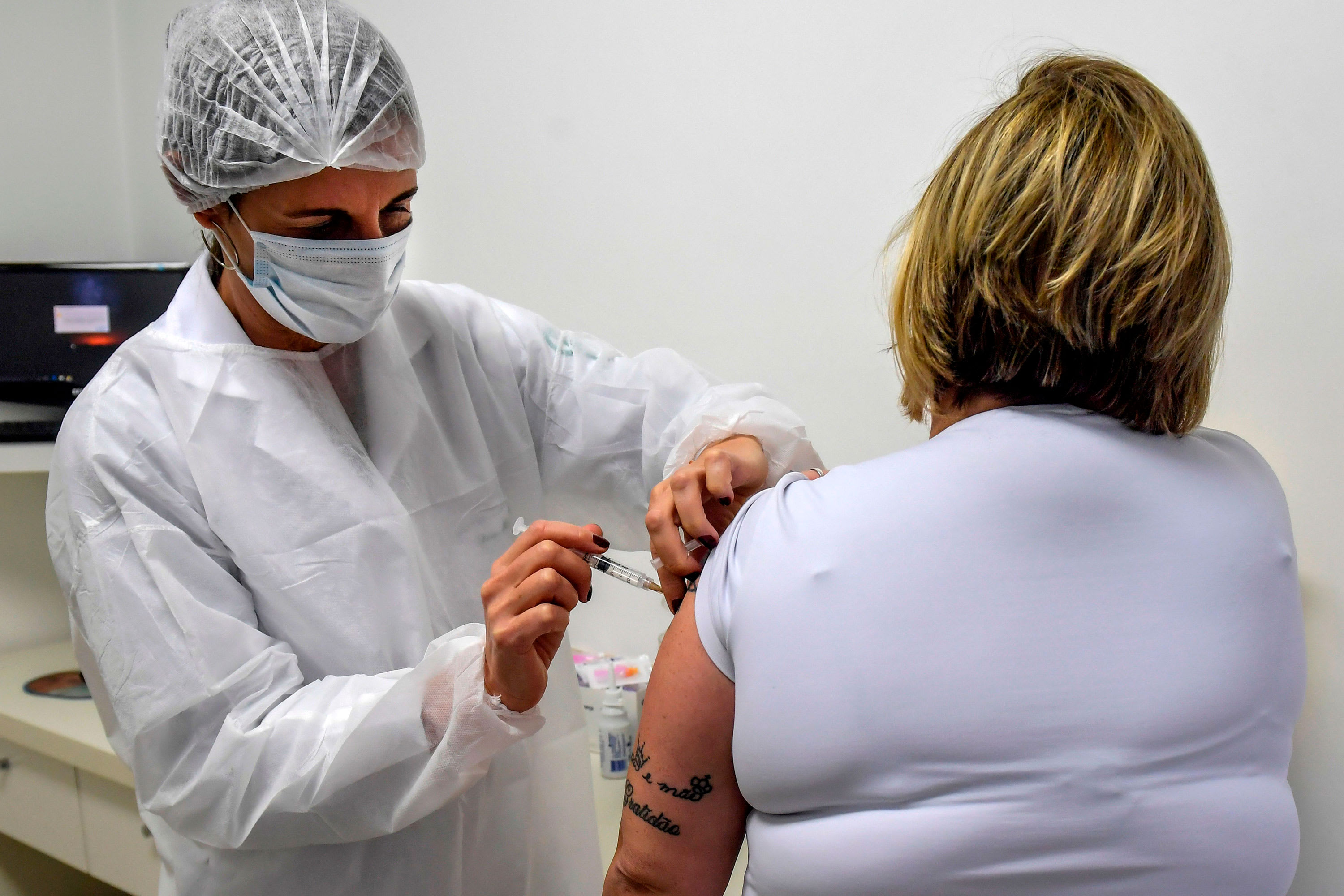
AstraZeneca’s movements turned negative on Wednesday after a Brazilian fitness authority, Anvisa, said a coronavirus vaccine volunteer had died.
The Federal University of Sao Paulo, which is helping coordinate complex level tests in Brazil, said the volunteer was Brazilian, according to Reuters.
The actions of AstraZeneca, the leader in the Covid-19 vaccine race, made a slight profit after the news was announced. Stocks fell by about 1% in the early afternoon.
An AstraZeneca spokesman refused to comment on the volunteer and mentioned “medical confidentiality and trial regulations. “
The spokesperson added that “[all] medical occasions are thoroughly evaluated through the trial researchers” and “[these tests did not lead to any consideration of the continuation of the ongoing study. “
In a statement, a spokesman for the University of Oxford, who is preparing the vaccine with AstraZeneca, said that “there has been no fear about the protection of the clinical trial” after a case evaluation in Brazil.
“The independent review in addition to the Brazilian regulator has to continue the trial,” said Oxford spokesman Alexander Buxton.
Oxford has not provided any additional important point about the volunteer’s death and it is unclear whether the volunteer won the vaccine. Brazil has recently had the deadliest outbreak in the world, the United States, with at least 115,914 deaths, according to knowledge compiled through Johns Hopkins University.
A person close to the stage told Reuters that the trial would have been suspended if the volunteer had been part of the organization that won the shooting.
The news comes when the Food and Drug Administration (FDA) still has a complex clinical trial of AstraZeneca pending in the United States, as the company cannot administer two-dose doses of its two-dose vaccine regimen to U. S. participants.
The company announced on 8 September that its trial had been suspended due to an unexplained illness in a patient in the UK. The patient is thought to have developed inflammation of the spinal cord known as transverse myelitis. Since then, the trial has resumed in the UK and other countries.
The United States is expected to resume this week after the FDA completes its review, Reuters reported Tuesday, and mentioned 4 unidentified sources.
AstraZeneca is one of 4 brands of US-backed drugs. But it’s not the first time In complex tests of a prospective vaccine. The AstraZeneca vaccine, called AZD1222, uses coronavirus genetics with a modified adenovirus.
In July, the company released the knowledge that its vaccine had produced a promising immune reaction in an initial trial and gave the impression of being well tolerated.
The vaccine did not produce any serious adverse occasions in the volunteers, according to the researchers at the time. Fatigue and headaches were the maximum side effects reported, they said. Other non-unusual side effects included injection site pain, muscle pain, chills and fever.
Do you have any confidential information? We want to hear from you.
Sign up for loose newsletters and get more CNBC in your inbox
Get it in your inbox and more information about our services.
© 2020 CNBC LLC. All rights are reserved. An NBCUniversal department
Knowledge is a real-time snapshot: data is delayed for at least 15 minutes, monetary and global industry news, inventory quotes, and market knowledge and analysis.
Data also by