The Southern Oregon Clinical Research Institute is the most recent medical organization that has conducted human trials for a leading candidate for the COVID-19 vaccine.
During the two years, the clinic’s director, Dr. Edward Kerwin, will act as lead researcher of the phase 3 human trials of the COVID-19 vaccine developed through the modern biotechnology company.
Kerwin is an allergologist and immunologist with 25 years of experience in clinical trials involving asthma, skin disorders and lung diseases.
The Medford clinic expects to recruit up to 700 volunteers until late August and will pay patients $1,962 for their participation.
Modern’s human trials will see 30,000 participants across the country at dozens of sites.
Another vaccine developed through Pfizer and BioNTech, a German company, is being tested lately on some 30,000 new recruits this year.
The clinic recruited patients from a variety of nearby workplaces in agriculture and food production where the threat of exposure to COVID-19 is greatest. It also seeks to recruit volunteers over the age of 65 who are at increased risk of serious illness or coVID-19 death.
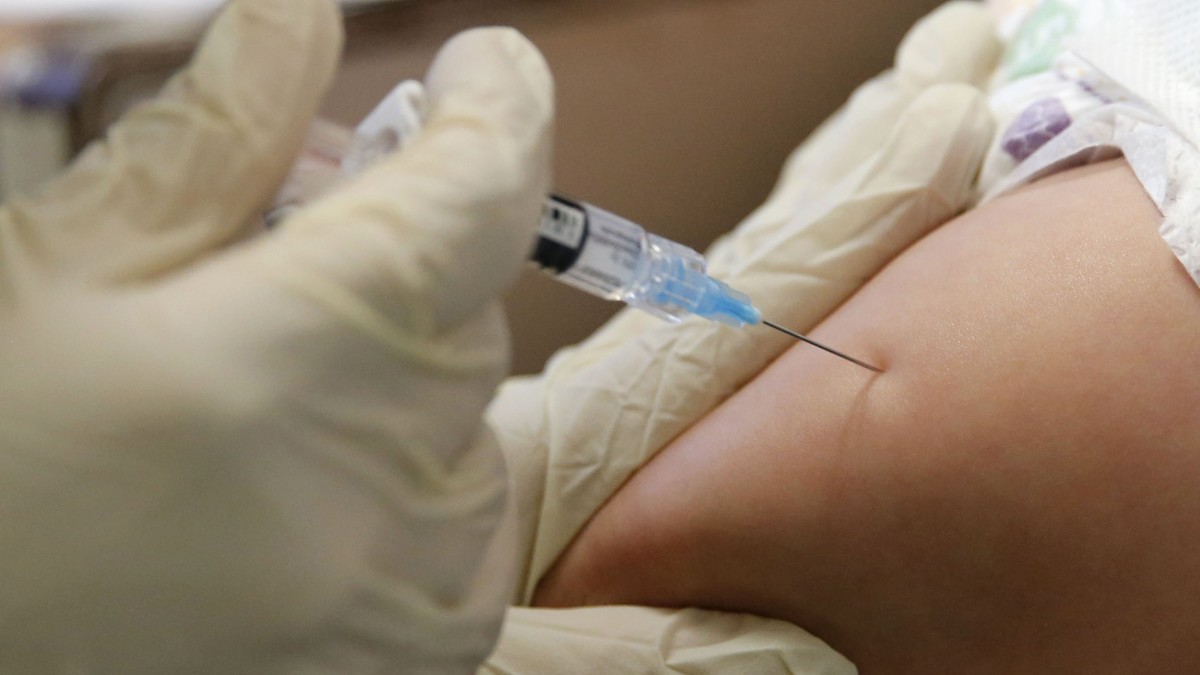
Half of participants will get two doses of the Modern mNR-1273 vaccine with a difference of approximately 28 days. The other part of the participants will get a placebo in the form of saline injection.
The vaccine uses a component of the COVID-19 genetic code that indicates that human cells produce a protein that develops an immune reaction to infection.
Study participants will keep a diary of their symptoms and undergo normal blood tests to see if they expand antibodies to the virus. Expected side effects of the vaccine include headache, fatigue, pain and redness at the injection site.
It takes two years to control the long-term effects of the vaccine.
On Monday, Jackson County, Medford, had 536 COVID-19 cases and a three-week average of 5.3%, even above Oregon Gov. Kate Brown’s 3-week threshold for the reopening of public schools.
Kerwin estimates that the Modern vaccine can be up to 90% effective.
The distribution of the vaccine in the United States and, in all likelihood, worldwide may take longer months, even if the trials are successful.
Moderna announced last week that it had ended a $1.5 billion contract with the U.S. government to produce one hundred million starting doses of its vaccine. The company’s human phase 3 testing began at the end of July. Preliminary effects are expected to end in September.
The CDC reported Monday that COVID-19 had inflamed more than 5.3 million people in the United States and killed 169,000 others.