Advertising
Supported by
Britain and Canada have a legal circular of recalls for immunocompromised senior citizens. So far, U. S. officials have not done the same.
Send a story to any friend.
As a subscriber, you have 10 gift pieces to offer per month. Everyone can read what you share.
By Apoorva Mandavilli
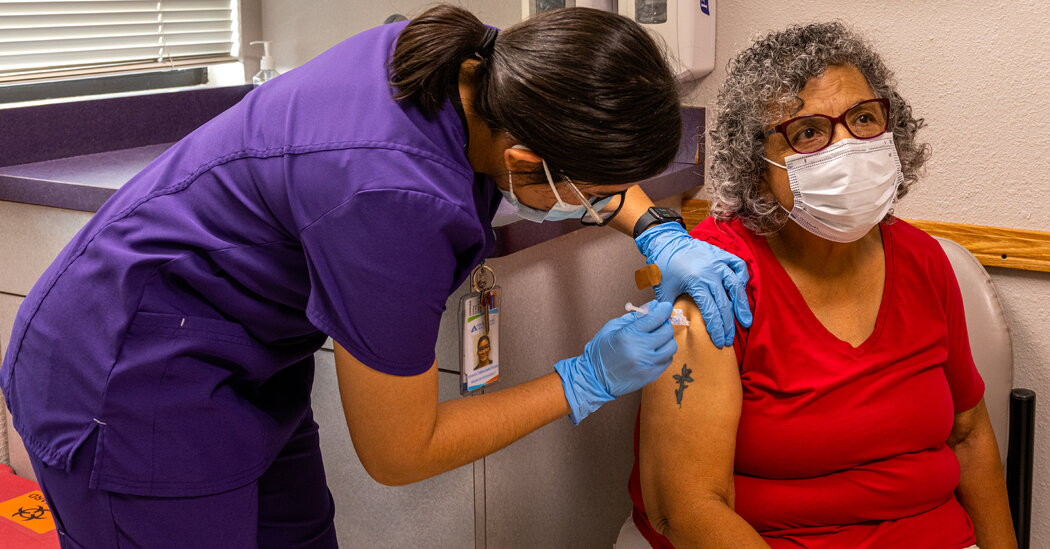
For most Americans, the coronavirus has a tolerable threat, on par with the flu, and requires minimal or no precautions. But for the elderly and the immunocompromised, the virus still poses a formidable risk.
In the United States, about three hundred people continue to die every day from covid-related causes, the vast majority of them adults over the age of 70 and others who are medically fragile or have weakened immune systems. Another booster shot now?
This is the thorny factor facing federal fitness officials.
About 53 million adults over the age of 65 in the United States, which is about 16% of the population, according to the Census Bureau. And seven million Americans have weakened immune systems due to illness or medication.
While coronavirus infection can be an inconvenience or mild illness for a young, healthy adult, covid can mean serious illness, hospitalization, and death for seniors and immunocompromised people, Dr. Brown said. Celine Gounder, infectious disease physician and senior scientist at Kaiser Family. Base.
“I think it’s moderate to stimulate other immunocompromised people and other people in nursing homes every six months,” Dr. Gounder said. “I don’t think annual reminders to everyone make sense. “
Some Americans who received their last booster in the fall ask their doctor when they will get the next dose. Britain and Canada have already recommended more shots for the elderly and other immunocompromised people starting this spring.
It’s unclear whether the Food and Drug Administration will stick to the lawsuit. In an effort to simplify what had a mind-boggling set of vaccination guidelines, the company said in January it would switch to a vaccine for singles introduced each fall to all Americans, as is the case with the flu.
“We expect that simplifying the COVID-19 vaccination schedule in the not-too-distant timeframe will lead to the vaccination of more Americans in the coming years,” the firm said in a statement.
One one-size-fits-all technique may not work. Americans “are varied in our age, we are varied in our risk, we are varied in our perception of risk,” said Dr. Ofer Levy, director of the precision vaccine program at Boston Children’s Hospital and an FDA adviser.
Ideally, Dr. Levy said, a high-risk user can simply consult a physical care provider and if they need an additional dose of the vaccine.
Immunity to infection decreases after only a few months in almost all other vaccinated people. But in other people with weakened immune systems, coverage for a booster shot in the fall “decreases temporarily enough that in the spring or summer they are no longer distinguishable from other people who haven’t gained a booster,” said Dr. Jeremy Faust, an emergency physician and fitness policy specialist at Brigham and Women’s Hospital in Boston.
“Other immunocompromised people have a very, very short era of booster benefit,” Dr. Faust said. “And then you have to upload it again. “
Dr. Faust cited knowledge of the first recall circular, which was filed in the fall of 2021 and focused on the original issue of the virus. There are far fewer studies on the effectiveness of bivalent boosters than were introduced last fall, and there is no knowledge. about the ideal time for the next circular of supplemental vaccines.
The lack of data makes some experts hesitant to introduce a vaccine for any group of people, even the most vulnerable.
“Given the lack of data, I don’t think it’s fair to tell people, ‘Inject a biologic,'” said Dr. Paul Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia and an FDA adviser.
“It is incumbent upon them, in giving advice, to show the knowledge on which that counsel is based,” Dr. Offit said of federal fitness officials. “Otherwise, they just say, ‘Trust us. ‘”
The FDA has not commented on plans to consider providing boosters more than once a year.
“We continue to closely monitor emerging knowledge in the U. S. We will base any resolution on more up-to-date drivers on this knowledge,” the firm said in a statement.
Even if the F. D. A. Si some other booster shot were authorized this spring, it’s unclear how many more people would receive it. Just over 16% of Americans and only 42% of adults 65 and older have opted for bivalent injections.
“If reminders work, they will only work if other people get them,” said Dr. Eric Rubin, editor-in-chief of the New England Journal of Medicine and an adviser to the FDA. “This is a much more important factor than a subsequent retirement. . “
Dr. Camille Kotton, who treats immunocompromised patients at Massachusetts General Hospital in Boston, said most of them are not up to date on their vaccinations. He cited many conceivable reasons: they are unaware of the recommendations, consider the data too confusing, or are simply willing to leave the pandemic behind.
“It’s okay to focus on some other dose of bivalent vaccine, but I’m involved in the fact that we haven’t even given the bivalent vaccine to most immunocompromised and elderly people,” she said. “Maybe we’ll focus on those populations. “
Advertising