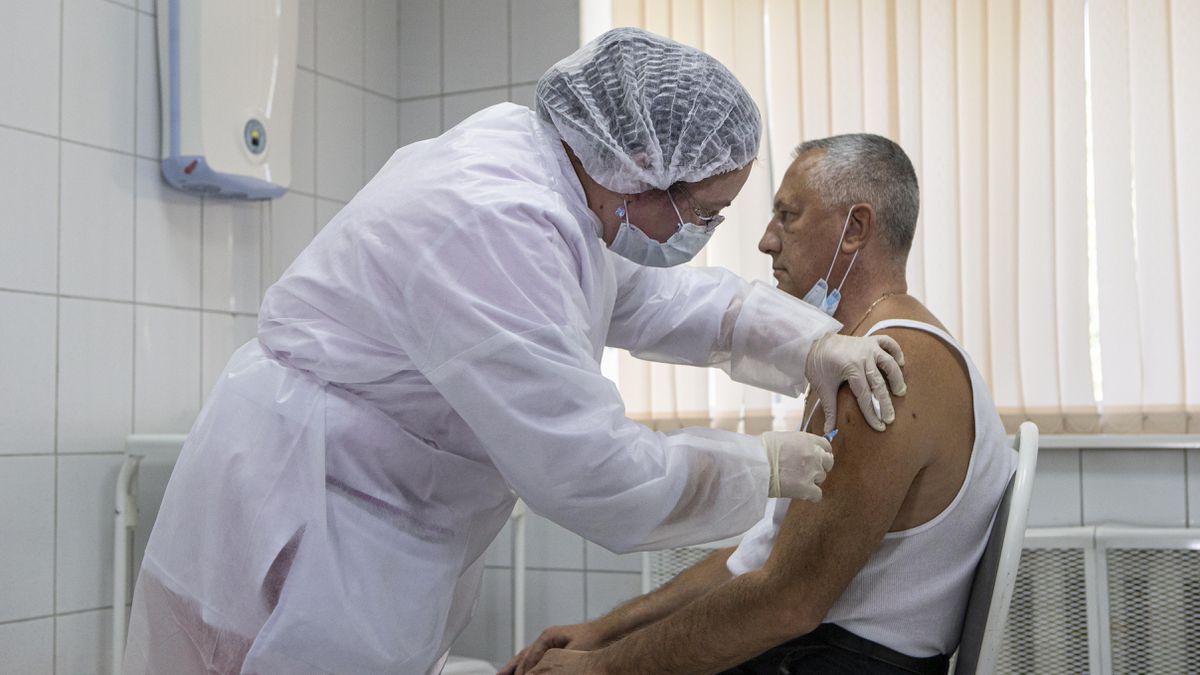
Russia has granted regulatory approval to the Covid-19 vaccine at a time, the drug has not yet begun large-scale Phase III trials, two months after the launch of another vaccine on which experts have expressed caution due to a lack of sufficient knowledge about protection and efficacy.
The moment the vaccine was announced through Russian President Vladimir Putin at a government assembly on Wednesday, Reuters reported.
The peptide-based drug, called EpiVacCorona, evolved through the Vector Institute in Siberia.
In a placebo-controlled trial, one hundred volunteers, between the age of 18 and 60, won the vaccine in the city of Novosibirsk.
The effects of these initial trials have still been published and large-scale Phase III trials, which help identify protection and efficacy, have yet to start.
Putin said Russia will increase the production of vaccines that have been approved by the government and plans to collaborate with “foreign partners” to publicize the vaccine abroad.
In August, Russia became the first country to grant regulatory approval to a Covid-19 vaccine, despite limited testing. This vaccine, called Sputnik-V, was developed through the Gamaleya Institute in Moscow. Scientists participated without the proper trial. However, the Russian president said the drug was safe and effective, noting that it had already been administered to one of his daughters. location that puts them at increased risk of inflammation with coronavirus have been vaccinated with the Sputnik-V vaccine, however, the vaccine is not yet widely used. When Sputnik-V was announced, former FDA communication Scott Gotlieb said Russia was “certainly not” ahead of the US. But it’s not the first time In the progression of the Covid-19 vaccine, and noted that the US is not the only one in the world to do so. But it’s not the first time It would not allow the mass distribution of a drug that had only been tested. in a few hundred patients at most.
213. This is the total number of newly developed Covid-19 vaccines. Only nine of them, adding the other Russian vaccine, Sputnik-V, have officially begun large-scale “phase III” clinical trials.
Earlier this month, WHO Director-General Tedros Adhanom Ghebreyesus expressed hope that a vaccine will be in condition until the end of the year; however, experts warned that the WHO Director’s schedule would possibly be too optimistic, noting that by the end of the year, there may be some kind of emergency approval that provides some availability to other high-risk people, such as fitness workers.
Russia approves COVID-19 vaccine after initial tests (Reuters)
Experts sound alarm when Putin says Russia has the world’s first Covid-19 vaccine (Forbes)
Comprehensive policy and updates on coronavirus
I’m a last-minute reporter at Forbes, with a policy of generating coverage and vital business news. Graduated from Columbia University with a master’s degree in business and
I’m a last-minute reporter at Forbes, with a policy of generating coverage and vital business news. Graduated from Columbia University with a master’s degree in economic and business journalism in 2019. I worked as a journalist in New Delhi, India, from 2014 to 2018. DVs are open on Twitter @SiladityaRay or email us at siladitya@protonmail. com.