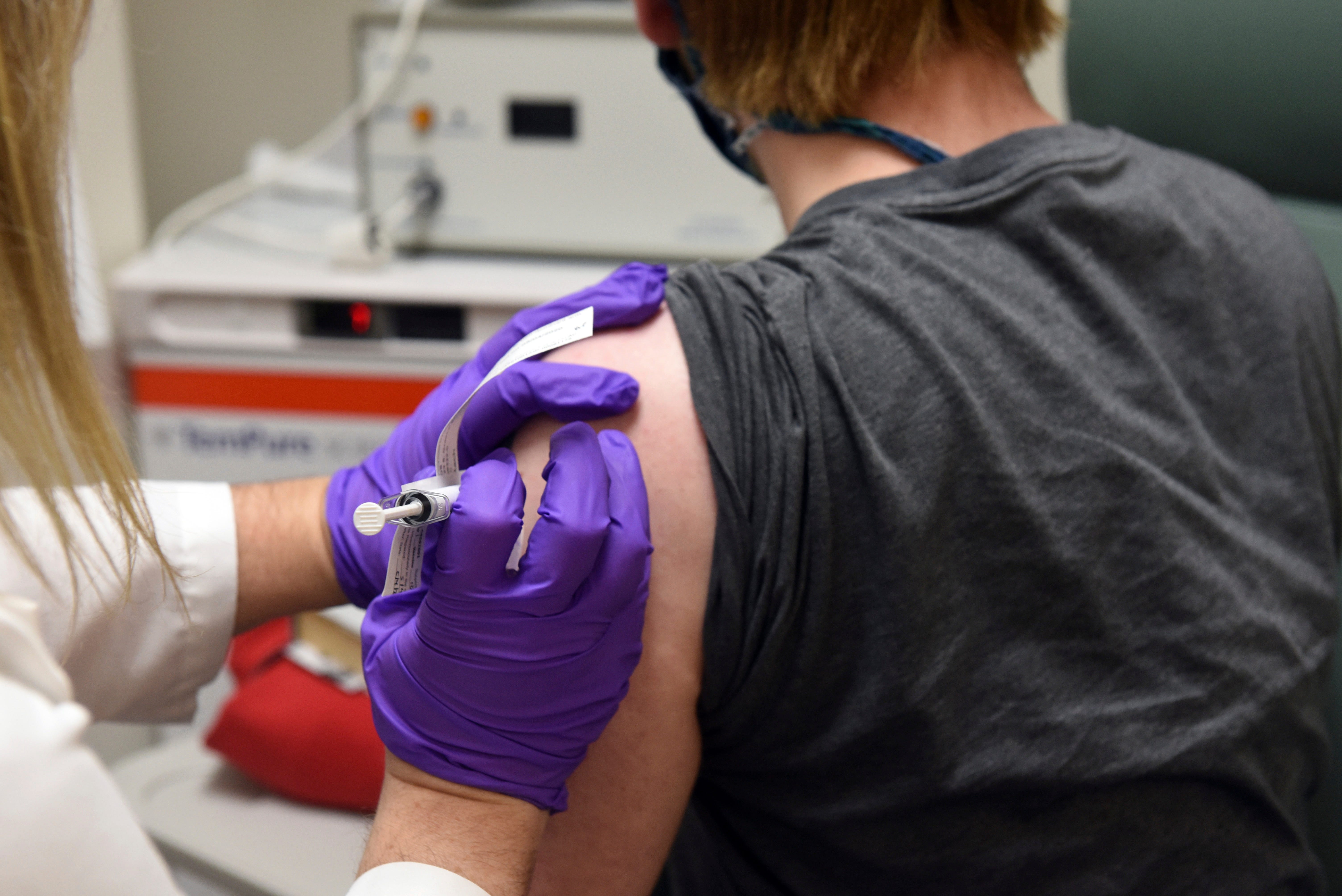
Pfizer, one of the pioneers in the search for a COVID-19 vaccine, said his vaccine candidate looked and that the company hopes to have knowledge next month about how he protects others from coronavirus.
Pfizer CEO Albert Bourla said Tuesday that he deliberately shows more data on the COVID-19 candidate vaccine than on any other progressive vaccine because the procedure must be open and transparent.
“Transparency is imperative, especially given this and the politicization of the vaccine,” he said in an interview with journalists.
The company said Saturday that it was expanding its trial from 30,000 to 44,000 more people to accompany adolescents, the elderly age of 16 to 18, as well as others with diseases such as HIV and hepatitis A, B or C. On Tuesday, Bourla said the expansion had taken a position because the vaccine seemed amazing and the trial could go on without delaying the final touch program.
Speed of light? Why a coronavirus vaccine is in over a year, despite advances through medical researchers
In knowledge published Tuesday, the company showed that players, both young and old, complained of minor side effects, such as headaches and arm pain. Knowledge included about 6,000 people; some won the active vaccine and others won the placebo. The company, which is preparing its vaccine, called BNT162, in collaboration with German vaccine developer BioNTech, does not know which player received which player received which one.
An independent knowledge safety commission knows who won the active vaccine and verifies that there are no fitness issues, Pfizer executives said.
There were probably serious fitness disorders in the trial, but the protection organization “concluded that none of them were vaccine-related,” said Kathrin Jansen, Pfizer’s senior vice president and head of vaccine studies and development.
The larger number allows the company to take a closer look at subgroups of participants, such as those with underlying fitness disorders such as HIV, and those who had the virus when they won the vaccine, he said.
A final report on the protection and efficacy of the vaccine will be in a position to be reviewed by regulators next month.
”This is unprecedented at all’: AstraZeneca’s COVID-19 vaccine trial stopped after the disease
The vaccine will need to be at least 50% effective – protective on average at least to some of the other people who take it – in order for federal approval to be discharged and given to the public. Pfizer manufactures BNT162 in 3 plants in the United States – in Kalamazoo, Michigan; St. Louis; and Andover, Massachusetts, as well as in Germany and Belgium.
It is not known how long the vaccine will be protected. The company is preparing for 3 possibilities: that other people want an annual vaccine, such as for the flu; they will want a vaccine every few years, as with tetanus; or that it will be unique, like the polio vaccine, said Dr. Mikael Dolsten, the company’s clinical director.
“We, this vaccine has the possibility of providing moderate and intelligent protection, but we want to monitor it,” Dolsten said. “It is also moderate to assume that we may want a seasoning in the future, as it is a genuine pandemic. “and there will be many viruses circulating even after the global [vaccination] campaigns. “
Jansen said that while Pfizer is engaged and “very satisfied with his existing vaccine candidate,” the company is already operating in a generation of times.
Pfizer hopes to make two innovations for the next generation, he said: the desire to keep the vaccine frozen and modify the generation so that a singles dose is desired, rather than two.
BNT162 uses a generation called messenger RNA, which trains a person’s cells to produce a protein on the surface of the coVID-19 coronavirus. Once the immune formula learns to recognize this protein, it will attack when you see it again in the virus.
Achieving herd immunity: how vaccines and masks are the keys to fighting the coronavirus
In a next-generation vaccine, Jansen said, she could be a messenger RNA that can also simply grow. “There would probably be an opportunity that with such an approach, you can also want only one injection” that would provide “the preparation and effect of stimulation. “
The vaccine should be kept frozen at minus-80 degrees, the temperature of the ice dry. Pfizer ships BNT162 in portable refrigerators that maintain ultra-cold temperatures. The fridge can be fed dry ice to keep the vaccine frozen for 15 days, then cooled up to five more days before being diluted and injected, said Angela Hwang, president of Pfizer Biopharmaceuticals.
Because the company expects the increased demand for the vaccine and its shipping network to be effective, Hwang said the vaccine will not be heat damaged. “No dose will stay for long, ” he said.
A second-generation vaccine requiring bloodless situations would be an improvement, Dolsten said.
Bourla, speaking at the end of a two-day investor conference, lamented the politicization of the COVID-19 vaccine and drug development.
But he said he believes the pharmaceutical industry is “up to the task” of the pandemic and expects it to be the public symbol of the industry.
“I don’t need to claim any victories, just because we’re passing by to bring a vaccine or a remedy that works, we’re moving to get back to where we deserve to be, but I think it’s going through being a very smart step. “”, he said. ” Then we have to keep doing the right thing. “
He had a fundamental idea at the end of the period: “Science will win,” he said.