“o. itemList. length” “this. config. text. ariaShown”
“This. config. text. ariaFermé”
Pfizer (PFE) and BioNTech SE have concluded exploratory discussions with the European Commission for a proposal to obtain two hundred million doses of their MRA162-based experimental vaccine candidate opposed to SARS-CoV-2 for the Member States of the European Union (EU). includes an option for another hundred million doses.
Deliveries are expected to begin at the end of 2020, a topic of clinical good luck and regulatory approval. Corporations will now begin contract negotiations with the European Commission.
The proposed source agreement with the European Commission would constitute the largest initial vaccine dose order to date for Pfizer and BioNTech (BNTX). Vaccine doses for Europe would be produced at BioNTech’s German production, as well as Pfizer’s production in Belgium. If regulatory approval for the BNT162b2 vaccine candidate is received, the European Commission will lead the procedure for assigning vaccine doses among the 27 EU member states.
“The planned agreement between Pfizer and BioNTech with the European Commission is a vital step in our shared purpose of making millions of doses of a COVID-19 vaccine available to vulnerable populations before the end of the year,” said Albert Bourla, CEO of Pfizer. “We have activated our chain of origin, especially our site in Belgium, and are starting to manufacture to make our vaccine available as temporarily as possible, if our clinical trials are successful and regulatory approval is granted.
The BNT162 program is based on BioNTech’s mNR generation and is backed by Pfizer’s global vaccine production and progression capabilities. On July 27, Pfizer and BioNTech decided that the BNT162b2 candidate vaccine would move to a phase 2/3 study. Code BNT162b2 for an optimized full-term glycoprotein (S) of SARS-CoV-2, which is the target of neutralizing antibodies to the virus.
In the complex trial, Pfizer and BioNTech are reading a 30 mg dose at a 2-dose regimen at up to 30,000 participants over the age of 18 to 85. Corporations decided on 120 sites worldwide, adding those from regions where SARS-CoV-2 transmission was expected to be significant.
Assuming clinical success, Pfizer and BioNTech are on track to request a regulatory review for BNT162b2 as of October 2020 and, if approved or approved, lately plan to supply up to one hundred million international doses by the end of 2020 and approximately 1. 3 billion doses by the end of 2020. the end of 2021.
To meet these expected quantities, corporations claim to have already produced enough materials for their phase 2/3 clinical trial for 30,000 participants and have begun producing and purchasing them on the occasion of a pandemic.
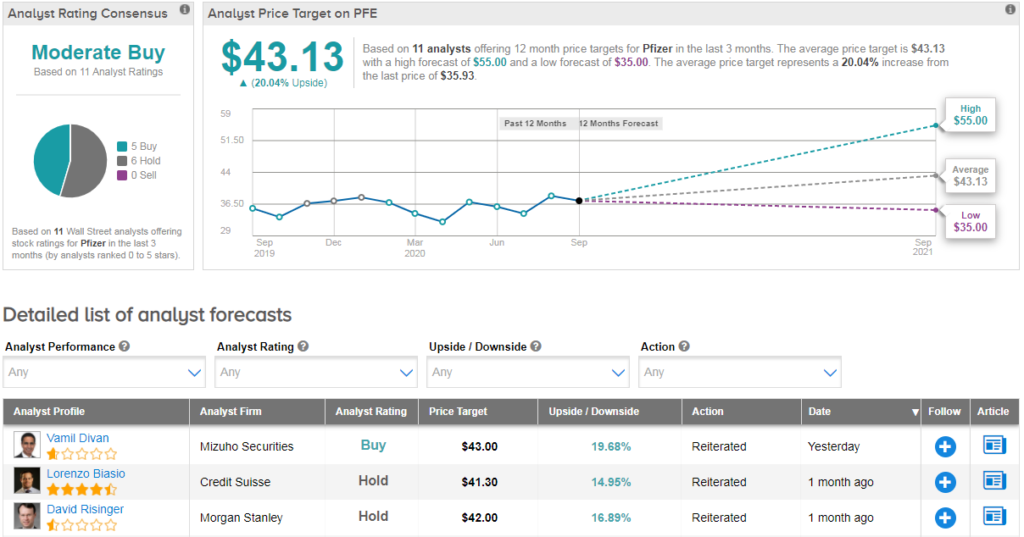
Pfizer’s inventories have dropped by 8% since the beginning of the year, and inventory is gaining a cautiously positive consensus on the moderate shopping street. The target analyst’s average value of $43 indicates a prospective increase of 20% of existing levels.
“We have been inspired by pfizer’s progress with the vaccine to date and are adding risk-adjusted sales of approximately $1. 7 billion in 2021 and approximately $1. 2 billion in our model. In 2022, which may be prudent given the most agreements signed through the company since the last update of our projections,” said Vamil Divan, an analyst at Mizuho Securities.
However, after the initial bolus, the analyst assumes a constant annual sales rate of $500 million to $600 million as of 2024. According to Divan, these limits “have an effect on which the vaccine would possibly have to help Pfizer through the post-2025 era during which the company will face a number of major patent expirations. It has an inventory score and a value target of $43 (see PFE inventory research at TipRanks).
Related News: Trillium: A recent weakness presents a buying opportunity, analyst Morgan Stanley says he turns his back on Eli Lilly and suspends PT AstraZeneca’s Covid-19 trial after an adverse reaction; Stock drop 8%
Billionaire David Shaw to pay in cash on 3 shares of ‘Strong Buy’
Village Farms buys remaining stake in Pure Sunfarms for $1 million
Activision Blizzard or Electronic Arts: What game name is in a position to win?
Dr. Reddy’s launches remdesivir for Covid-19 remedy in India