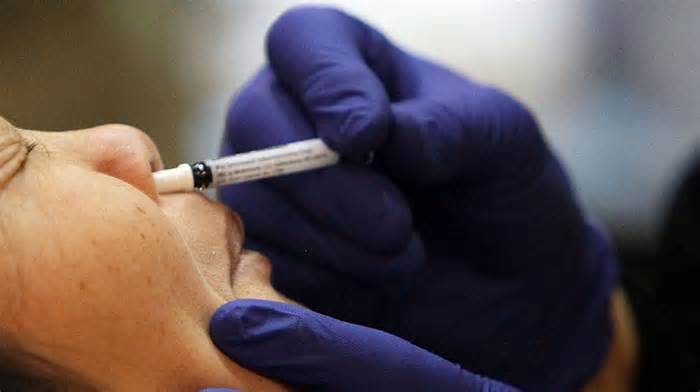
The University of Health Sciences (UHS) has to organize clinical trials on a nasal vaccine opposed to COVID-19 developed through a Chinese company.
Depending on the details, clinical trials will begin in six to eight weeks for approval through 3 committees.
While talking to the media about development, UHS Vice-Chancellor Dr. Javed Akram said the first approval of the UHS Review Committee will be required, followed by momentary approval from the National Bioethics Committee. grant final approval to UHS for clinical trials of the COVID-19 nasal vaccine.
He added that the COVID-19 nasal vaccine is a singles dose manufactured through CanSino Biologics, whose intravenous singles dose vaccine is already in use in Pakistan.
About 5,000 volunteers will participate in UHS clinical trials on the efficacy and protection of the COVID-19 nasal vaccine, Akram noted, adding that volunteers would be evaluated with PCR testing and next-generation sequencing to analyze the effectiveness of the vaccine.
Dr. Akram expressed the hope that the vaccine will generate the expected immune reaction at the time of infection and in the airways, as the vaccine is already used in China.
Behavior
The query has expired