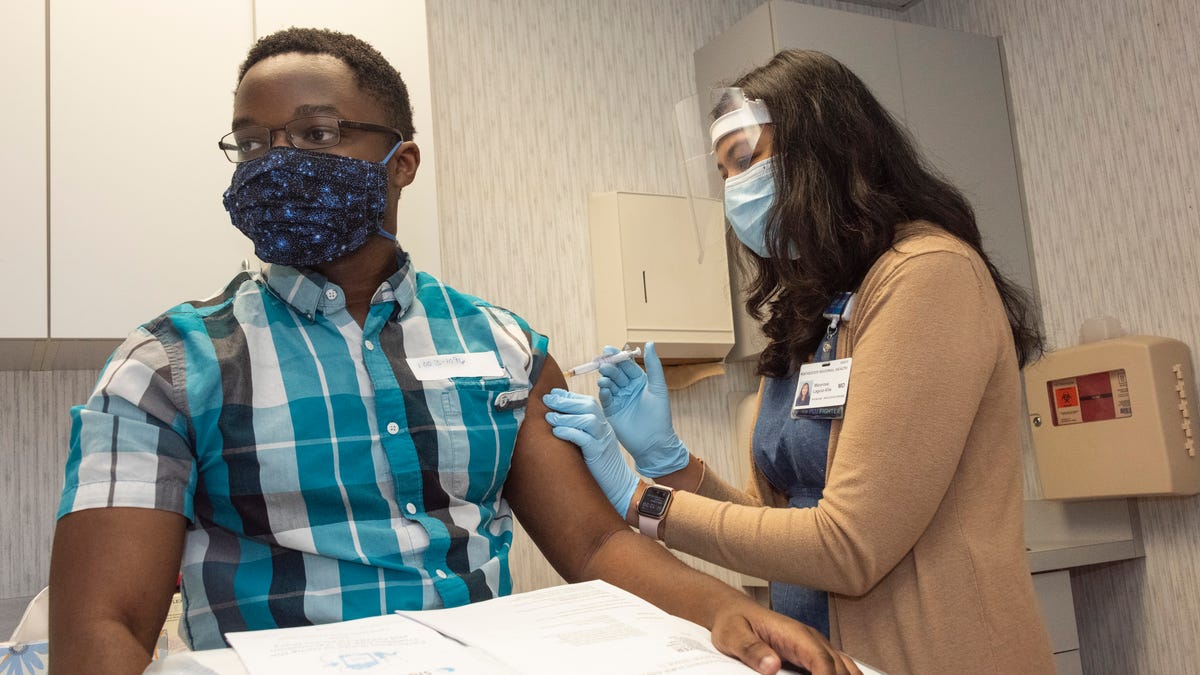
Sixteen other people in the Rochester domain are among the thousands of volunteers who participate in a primary coronavirus vaccine trial has been suspended.
The plan required the participation of approximately 1,000 local people in the trial.
The Anglo-Swedish pharmaceutical company AstraZeneca, which is preparing the vaccine in collaboration with the University of Oxford, announced this week that it would discontinue global Phase III trials of its vaccine because a player had evolved”. . . an inexplicable disease. “
None of the other 16 people who have so far won an injection into Rochester’s domain have reported “unexpected or severe reactions,” the University of Rochester Medical Center said on a Wednesday.
“According to normal exam protocols, medical center researchers will continue to monitor the fitness of everyone taking the test on a daily basis,” the URMC said.
UR is one of 80 sites in the United States that started phase III trials of the vaccine in August. Trials are also underway in the UNITED Kingdom, Brazil and South Africa.
More than 4,000 adults in the Rochester domain have volunteered to participate in coronavirus vaccine studies, URMC spokesman Mark Michaud said.
Stopping the AstraZeneca trial has no effect on studies of other vaccines, one of which is being developed through Pfizer and BioNTech.
URMC and Rochester Regional Health are jointly a phase 3 trial for this vaccine. Approximately 120 other people won the vaccine or a placebo in the Pfizer trial, according to Michaud. Another 48 people took part in an earlier Pfizer test here.
URMC researchers began recruiting participants on the AstraZeneca exam on August 2 and administered injections to 16 others before closing. Michaud said the records will resume when he does the biggest test.
Some trial participants get the vaccine, while others get a placebo. They will need to receive two injections 4 weeks apart, with a monitoring of their fitness for two years.
The goal is to practice a giant and varied organization of vaccine receptors to assess whether the vaccine is effective in protecting against coronavirus and whether it is safe. A group of participants in the early stages of the AstraZeneca trial did not yet discover serious side effects. many minor effects such as headaches, chills and muscle aches.
The New York Times reported that a case of transverse myelitis, of the spinal cord, had been provisionally diagnosed in a player in a vaccine trial in the UK.
It is still known if the condition is vaccine-related.
Contact surveillance reporter Steve Orr at sorr@democratandchronicle. com or (585) 258-2386. Follow him on Twitter at SOrr1. This policy is only imaginable with the help of our readers. If you don’t already have a virtual subscription, sign today.