The Dallas Research Institute has recruited the world’s first patients to test a new manufactured antibody.
DALLAS – A new clinical trial of Operation Warp Speed is now underway at Baylor Scott and White.
The institute of studies has recruited the world’s first patients to test a new manufactured antibody.
The Baylor Scott-White Research Institute has the first site in the world to start testing a new type of antibody manufactured in a laboratory in hospitalized patients.
And while the test is a component of Operation Warp Speed, the director says there will be no shortcuts to get answers.
Dr. Uriel Sandkovsky leads the new trial at Baylor Scott-White in partnership with the National Institute of Health.
“To be offering hope to those who walk through the door, I think it’s the ultimate vital part,” he said.
Advertising
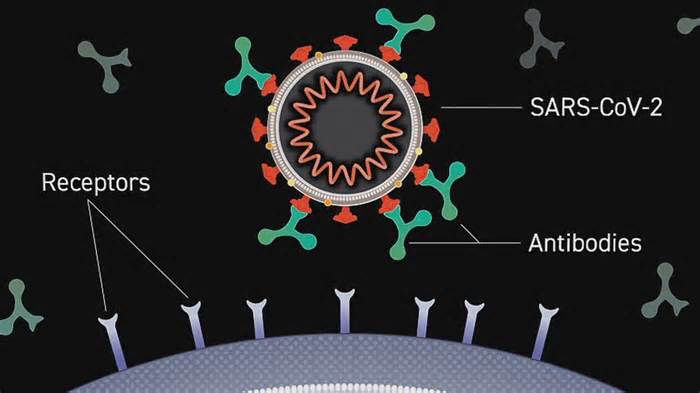
It is an antibody manufactured in a laboratory for inpatients. A monoclonal antibody attaches to the coronavirus, blocking its access to a human cell.
Unlike maximum trials, in this case, if the antibody drug does not work, Baylor Scott-White may temporarily adapt to bureaucracy.
“We are allowed to introduce some other remedies as a component of the trial,” Dr. Sandkovsky said. “If an antibody doesn’t work, we can check an antibody from some other company for use in our patients.”
Trial patients will not know if they are receiving a monoclonal antibody or placebo. All patients will continue to receive remedesivir antiviral, the popular existing hospital remedy for COVID-19.
“We know that one hundred percent deal with other people and not guinea pigs. The goal is to help others,” the doctor said.
Dr. Sandkovsky says that whether he discovers a life-saving drug in Dallas or not, he knows that the pieces of the remedy puzzle will be discovered.
“It’s a team effort around the world. We’re part of it. We have to be humble,” he said.
The only patients eligible for the clinical trial are those who require hospitalization. So far, five patients have volunteered to participate in the trial in Dallas.