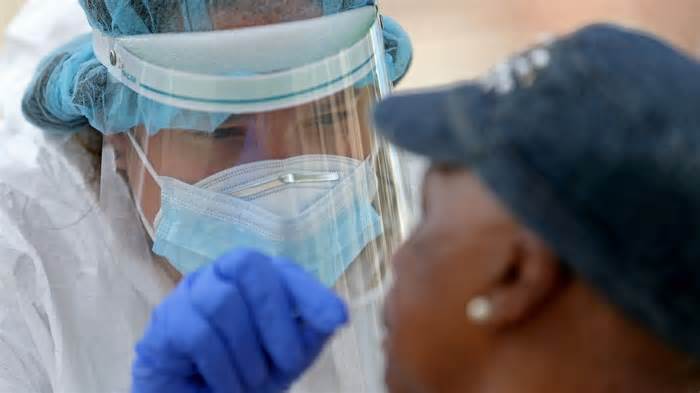
To prevent the furious coronavirus epidemic, which is recently spreading at a rate of about 60,000 new instances per day, the United States can do one of two things.
You can block them all, or you can check them all constantly.
The lockdown approach works because if infected people can’t get close to noninfected people, the chain of transmission is broken, even if we don’t know who is actually infected.
The verification technique works because it allows us to identify other inflamed people and isolate them from other inflamed people without forcing everyone to stay home.
But the problem is that after enduring a great national this spring, and suffering the economic consequences with little to show, Americans have no appetite even for specific, localized aftermath.
That leaves evidence.
Unfortunately, the most recent knowledge shows that evidence is declining in much of the United States. According to Johns Hopkins University, the average number of COVID-19 tests consistent with the consistent form of another 1,000 people has decreased during the following week in 30 states, more than part of the country. Nationally, the average number of tests fell by 8.75% during the same consistent period, from 822,470 on July 29 to 750,517 on August 4.
And while some of the states where the tests failed were affected by Hurricane Isaiah, Maximum did not. Most are states, 22 in total, where a higher percentage of COVID-19 tests test positive, indicating that they do not show a wide enough network to track (and control) their epidemics.
In short, while President Trump is right to say that the United States has conducted more tests than any other country, it is not testing enough, given the scale of the epidemic. And the evidence he’s doing, for reasons we’re going to explain, helping as much as he should.
The good news is that there may be an undeniable solution: new tests that prioritize speed over sensitivity.
In addition to storms, the main explanation for why U.S. checks are shrinking rather than expanding is that the type of verification we perform (PCR (chain reaction through polymer). PCR checks are the popular gold for diagnosing COVID-19, and rightly so: they identify more than 98% of positive cases.
But they are also slower and slower. As the virus spreads, more and more potentially exposed Americans are not easy to control, forcing defeated U.S. labs to compete with other countries for the materials needed to process so many samples. The labs ran out. As a result, crippling delays have delayed the effects of checks for so long that they are worthless.
According to a new national survey conducted through researchers from Harvard University, Northeastern University, NorthwestEr’s University and Rutgers University, Americans evaluated for COVID-19 in July reported that they waited an average of 4 days to get their results. About 10% of other people said they waited 10 days or more.
However, public fitness experts say the results, which take more than 24 to 48 hours to arrive, disagree with the verification goal. By the time other people get a positive result, they may have already inflamed other people. By the time they get a negative result, they may have become inflamed because of others.
Americans seem to perceive the message. “Long wait times and long response times mean that other people are simply giving up,” said Dr. Ashish Jha, director of the Harvard Global Health Institute, Tuesday.
The verification cave in the United States has revealed, in short, its fatal flaw. Costly and bulky PCR checks are appropriate when an epidemic is relatively small; In this situation, you can invest in slower, more labor-demanding control that will not lose any positive cases, because you need to prevent the virus from spreading before it is too late.
But when it is too late, when more than 4.8 million infections have been reported and only about 160,000 more people have died, PCR infrastructure cannot remain active.
That’s why experts like Dr. Michael Mina, assistant professor of epidemiology at Harvard Medical School and Harvard T.H. Chan School of Public Health, we’re abandoning it and starting over.
“We need to change the whole script of what it means to test people,” Mina recently explained.
Imagine a $1, at-home, paper-based test that’s as easy to distribute and use as a pregnancy test. Imagine waking up in the morning, adding saliva or mucus to a tube of chemicals, waiting 15 minutes, dipping a paper strip in the tube and reading your results — instantly.
Now believe that every single user in the United States does this every single day.
So-called immediate antigenic controls are not science fiction. In fact, they already exist. Two of these checks, conducted through BD and Quidel, obtained emergency approval from the Food and Drug Administration, and still require tools to operate. (Governors in six states announced this week that a team will offer to acquire a total of 3.5 million of those antigen controls.) Another $1 antigen control has been implemented in Senegal. And U.S. corporations like E25Bio and Sherlock Biosciences have developed housing controls as reasonable and simple as those described above.
These are not the same antibody tests you may have heard of, which stumble upon antivirus ingredients in the blood of others that have been swollen in the past (and can be immunized). Rapid antigenic testing is designed to trip over active infections in progress.
So why the U.S. government Does it not produce antigenic mass tests and distribute them to all? The biggest impediment so far has been what reports tend to call “precision.” But “sensitivity” is a better way to think about it.
PCR tests make thousands of copies of the virus’s RNA and may trip over it to very low degrees. Antigen tests are based on a molecule that binds to the picos of coronavirus proteins; at very low levels of infection, there might not be enough viral remains in a sputum pattern to cause a positive result.
The concern is that if we rely on antigenic testing, we lose many cases. But there are two reasons why this concern would possibly be unfounded.
The first is that we are already missing tons of instances. According to CDC antibody data, our existing PCR formula checks only other people to trip over about 10% of the total number of infections. “If everyone took an antigen check, even if we only knew 50% of the positives, we would still identify 50% of all infections in the country,” Jha explained. This is “five times more than 10% of the instances we’re probably identifying, because we’re reviewing very few people.”
The time is that the amount of coronavirus in the picture increases exponentially in the first few days of infection. Initially, the incubation period, no control would be sensitive enough to trip over it. About 3 to five days later, a PCR test captured it. After another 8 to 24 hours, according to Mina, a quick antigen analysis would show a positive result.
It’s not about whether an antigen check would run into an infection. That’s when
That’s a very important distinction. Suppose PCR verification verifies a positive result on the 4th day and the antigenic check delivers a positive result on the 5th day. As far as experts know, days 4 and 5 are before or near the beginning of the window, that other people can transmit the virus to others. But if the user taking control of PCR does not know if it is positive until the eighth day or even the 14th day, while the user taking antigenic control is discovered on the fifth day, antigenic control is much more useful. to prevent the spread of the virus, even if it is less sensitive.
What happens if your viral load is too low on the fifth day for antigenic detection to detect it? Your infection may not yet be very transmissible. Then the next check that passes, the seventh day, would pick it up. And the user who took the PCR test still wouldn’t have the results.
“The vast majority of the positive PCR tests we’re recently collecting in this country run into other people long after they’ve stopped being infectious,” Mina recently explained. “All we do with all those tests is obstruct the testing infrastructure, and essentially locate other people we can’t even act for because they’ve finished transmitting.”
Current FDA rules state that any new coronavirus checkup that competes for emergency approval will have to be almost as effective as a PCR checkup. But as autumn approaches, and in colder weather, increased domestic activity, a return to school, and the possibility of even greater waves of infection, it may be time to reconsider those regulations.
Rapid antigenic testing has its challenges. A fair distribution on a giant scale would likely require billions of dollars of government investment; Easy and reasonable house tests would only paint if everyone takes them thoroughly.
But experts say they’re starting to make more sense than the quo.
“If you had asked me this question a few months ago, I would have told you to simply do the PCR tests,” Susan Butler-Wu, a clinical microbiologist at the University of Southern California, told the New York Times on Wednesday. “But we are so far away in this country. It’s a mess. It’s time for the kitchen sink.”
_____
Learn about Yahoo news:
Exclusive: CDC projects the number of coronavirus deaths in the United States may exceed 180,000 to August 22
Yahoo News/YouGov poll: Most of Trump’s electorate says they might not settle for the effects of 2020 if Biden wins because of mailed ballots
Fitness percentage classes in New York front line COVID-19
What is voter fraud? Yahoo News explains
Learning modules: an option for school?