n n n ‘. concat(e. i18n. t(“search. voice. recognition_retry”),’n
SPIKEVAX® XBB. 1. 5 is the first updated COVID-19 vaccine to be legal to use in Canada by fall 2023
Provinces and territories will have access to updated COVID-19 vaccines for their respective fall vaccination campaigns.
TORONTO, Sept. 12, 2023 /PRNewswire/ — Moderna Biopharma Canada Corp. Health Canada today legalized its updated COVID-19 vaccine, SPIKEVAX® XBB. 1. 5, to save COVID-19 in Americans 6 months of age. Now approved, Moderna’s updated COVID-19 vaccine will be available in provinces and territories to coordinate their fall vaccination campaigns.
“COVID-19 continues to pose a serious health riskii. With the authorization of our updated vaccine, Canadians have a vital tool to protect themselves, especially as we approach the peak of respiratory virus season,” said Shehzad Iqbal, Country Medical. Director, Moderna Canada. The National Advisory Committee on Immunization (NACI) recommends that anyone who is unvaccinated or has not contracted COVID-19 in the past six months receive an updated dose, focusing on protecting the vulnerable. ” Iii
To ensure that the updated vaccines provide the most physically potent immune reaction against the dominant circulating variants, regulators and global public health agencies are requiring COVID-19 vaccines to be upgraded to an XBB. 1. 5 monovalent composition. Health Canada contained clinical data showing an immune reaction against the XBB sublineages of SARS-CoV-2, adding XBB. 1. 5 and XBB. 1. 16.
In addition to the insights included in the presentation, Moderna has recently released more insights into clinical trials related to the ongoing evaluation of SPIKEVAX XBB. 1. 5 compared to circulating subvariants such as BA. 2. 86, EG. 5, and FL. 1. 5. 1. In August, Moderna announced additional clinical trial data showing that the company’s updated COVID-19 vaccine elicited a human immune reaction contrary to EG. 5 and FL. 1. 5. 1. iv. Last week, the company shared additional clinical trial data confirming that the updated COVID-19 vaccine also elicited a human immune reaction opposite to BA. 2. 86. v
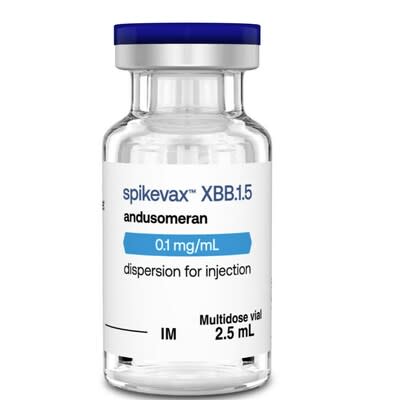
This authorization includes two key updates to streamline provincial vaccination management and systems. SPIKEVAX XBB. 1. 5 will be available in a single form (0. 1 mg/mL, 2. 5 mL vial) that will allow collection of adult and pediatric doses from the same vial. The dose has also been adapted to simplify management considerations, which require only the patient’s age and vaccination history to determine the appropriate dose.
About Moderna Biopharma Canada Corp.
Since its inception in 2010, Moderna has grown from a research-stage company developing messenger RNA (mRNA) systems to a company with a diverse clinical portfolio of vaccines and therapeutics in seven modalities, a broad portfolio of intellectual assets, and an integrated approach. amenities that enable immediate clinical and advertising production at scale. Moderna’s mRNA platform builds on ongoing advances in fundamental and implemented mRNA science, delivery technology, and manufacturing, and has enabled the progression of therapies and vaccines for infectious diseases, immuno-oncology, and rare diseases. , cardiovascular disease, and autoimmune diseases. More recently, Moderna’s features have been combined to enable the legal use and approval of one of the earliest and most effective vaccines against the COVID-19 pandemic.
Moderna has built relationships with government and business partners, leading to innovative science and an immediate increase in production. In Canada, Moderna has entered into a 10-year strategic partnership with the Canadian government to develop pandemic response capabilities, including the structure of a complex mRNA production facility for respiratory vaccines that is recently being built in Quebec. Moderna is also committed to helping position Canada as a leader in mRNA studies and progression through partnerships across the country. For more information, visit www. modernatx. com/en-CA
References:
I
SPIKEVAX® XBB. 1. 5 (2023) Product Monograph, Moderna.
I
Government of Canada: COVID-19 Epidemiological Update Summary: https://health-infobase. canada. ca/covid-19/. Retrieved September 8, 2023.
Iii
July 11, 2023 National Advisory Committee on Immunization Statement: Guidance on the Use of COVID-19 Vaccines in Fall 2023: https://www. canada. ca/fr/sante-publique/services/publications/vaccins -immunisation /national-advisory-committee-immunization-summary-11-july-2023-guidance-use-covid-19-vaccines-fall. html. Retrieved September 8, 2023.
IV
medRxiv: Safety and immunogenicity of XBB. 1. 5-containing mRNA vaccines. https://www. medrxiv. org/content/10. 1101/2023. 08. 22. 23293434v2. I consulted September 10, 2023.
v
Moderna Press Release: Knowledge of Moderna’s clinical trial confirms that its updated COVID-19 vaccine generates a strong human immune reaction contrary to BA. 2. 86. https://investors. modernatx. com/news/news-details/2023/Moderna -Datos-de-ensayo-clínico-confirman-su-vacuna-Covid-19-actual-genera-respuesta-inmune-fuerte-en-humanos-Contre-BA. 2. 86/default. aspx. September 6, 2023.
SOURCEModerna, Inc.
View content to download media: http://www. newswire. ca/en/releases/archive/September2023/12/c8914. html