By the time of this month, there are promising news of a vaccine candidate opposed to coronavirus disease 2019 (Covid-19): Moderna said Monday that his injections presented protection, a trail of hope in the dark context of coronavirus outbreaks in the United States. and all over the world.
Moderna said his vaccine appears to be 94. 5% effective, according to initial knowledge of the company’s ongoing study. A week ago, competitor Pfizer Inc. announced that its own Covid-19 vaccine also gave the impression of being effective, a news story that puts corporations on track to seek approval within weeks of emergency use in the United States.
Dr. Stephen Hoge, president of Moderna, welcomed the “really vital milestone,” but said it was very reassuring to achieve similar effects from two other companies.
“This deserves to give us all hope that a vaccine will be able to prevent this pandemic and bring us back into our lives,” Hoge told The Associated Press.
“It will not be Modern who will solve this problem. It will take a lot of vaccines” to meet global demand,” he added.
A vaccine cannot arrive fast enough, as cases of viruses exceeded 11 million in the US. But it’s not the first time This weekend, 1 million of them checked in last week. The pandemic has killed more than 1. 3 million people worldwide, adding more than 245,000 people in the United States.
However, if the Food and Drug Administration authorizes the emergency use of Moderna or Pfizer applicants, materials will be limited and rationed before the end of the year. Both require others to get two injections, several weeks apart. 20 million doses destined for the United States until the end of 2020. Pfizer and his German wife BioNTech expect to have around 50 million international doses until the end of the year.
The Moderna vaccine, created with the National Institutes of Health, is being studied in 30,000 volunteers who have won genuine vaccination or a fake vaccine. On Sunday, an independent follow-up committee broke the code to read about 95 infections recorded two weeks after the volunteers’ momentary dose, and found that all five diseases occurred in participants who won the placebo.
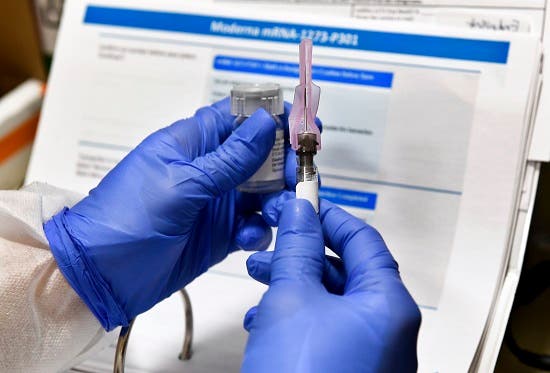
In this July 27, 2020 file photo, Nurse Kathe Olmstead prepares a photo that is a component of a imaginable COVID-19 vaccine, developed through the National Institutes of Health and Modern Inc. , in Binghamton, New York. AP PHOTO
The test is ongoing and Moderna has stated that the coverage rate will likely replace as more Covid-19 infections are detected and added to the calculations. Also, it’s too early to know how long coverage lasts. Both warnings also apply to Pfizer Vaccine.
But Moderna’s independent monitors provided additional and promising information: the 11 severe cases of Covid-19 worried placebo receptors and there were no protection issues.
The main side effects were fatigue, muscle pain and pain in injection after the vaccine dose, at rates hoge described as more common than flu shots, but comparable to others such as the shingles vaccine.
The Cambridge, Massachusetts-based vaccine is one of 11 community applications worldwide, adding 4 in massive US studies.
Modern injections and the Pfizer-BioNTech candidate are mNR vaccines, a new logo technology. They are not made with the coronavirus itself, which means that there is no chance of someone contracting it through fire, but that the vaccine consists of one piece. that causes the immune formula to recognize the enriched protein on the surface of the virus.
The smart effects were a surprise. Scientists have warned for months that any vaccine that opposes Covid-19 can be as smart as flu shots, which are about 50% effective.
Another primary challenge is distributing doses that want to stay very cold. Modern and Pfizer plans are frozen but at other temperatures. Moderna announced Monday that once thawed, its doses can last longer in a refrigerator than planned, up to 30 days. Pfizer snapshots require a long-term garage at extremely cold temperatures.
It has effectively joined our subscriber list.