“r.itemList.length” “this.config.text.ariaShown”
“This.config.text.ariaFermé”
Moderna showed that it participated in talks with Japan’s Ministry of Health, Labour and Social Welfare (MHLW) to potentially acquire 40 million or more doses of its mNR-1273 vaccine candidate opposed to COVID-19.
Under the terms of the agreement, the vaccine would be provided through Modern (MRNA) and distributed in Japan through Takeda Pharmaceutical Co., Ltd.from the first part of 2021, if the vaccine receives regulatory approval and is effective.
MHLW Minister Kato Katsunobu announced talks at a media meeting in Japan friday, when Japan is seeking to supply vaccines to the public as soon as possible, while Japan has already signed agreements with other drug brands such as Pfizer and AstraZeneca.
Last week, Moderna announced that it had concluded complex exploratory discussions with the European Commission to supply 80 million doses of mNRA-1273.Earlier this month, the U.S. government committed $1.5 billion to get a hundred million doses of its COVID-19 candidate vaccine.
MSAR-1273 is an mNR vaccine opposed to COVID-19 that encodes a prefusion stabilized form of the Spike protein (S), and is jointly developed by Moderna and researchers from the Vaccine Research Center of the National Institute of Allergy and Infectious Diseases (NIAID).
On Thursday, Moderna revealed that its mNR-1273 vaccine on the Phase 1 exam demonstrated physically powerful immune responses in older adults that were comparable to those of younger adults.
The corporate also announced that the Phase 3 COVE of mSR-1273 had begun on July 27; registration is nearing finishing touch in September.As of August 25, 15,239 participants were registered.
Modern reiterated that it is still on track to deliver approximately 500 million doses consistent with the year and up to one billion doses consistent with the year, starting in 2021.The initial investment of $1.3 billion for Moderna to produce mRNA-1273 was publicly provided in May 2020.
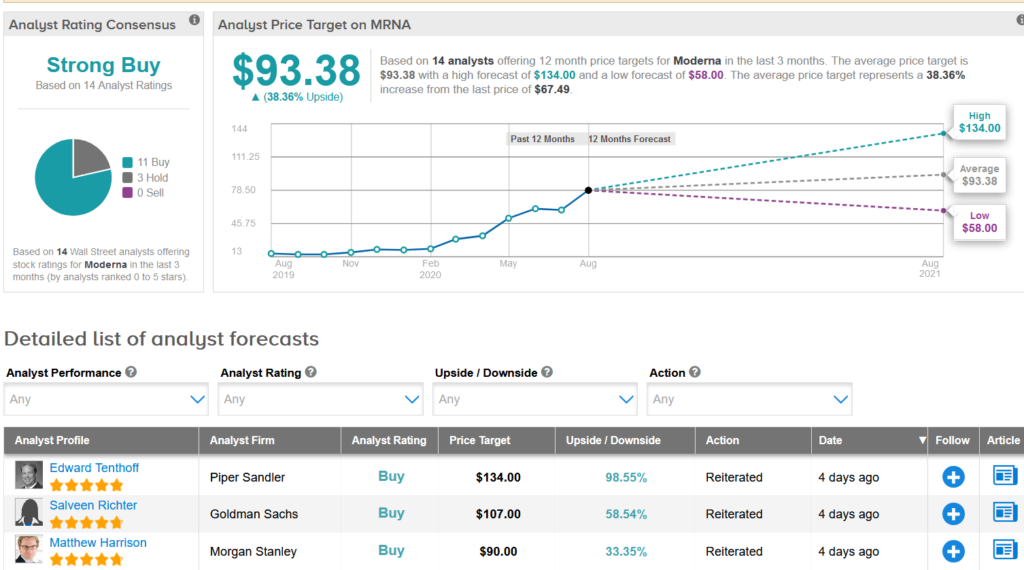
Moderna’s stock has fallen by 9% in the last month, but has soared 245%, so this year Wall Street analysts still have a Strong Buy consensus on the outlook for stocks.develop potential.
Commenting on the latest knowledge of Phase 1, JPMorgan analyst Cory Kasimov said the candidate vaccine appears to show a “promising profile,” you still need to see “the effects of Phase 3 with mRN-1273, which are expected this fall, to see how knowledge evolves.»
Kasimov maintains a Keep in Inventory score because he is skeptical of “Moderna’s ability to generate significant long-term revenue that justifies prevailing market values.”
Related news: Modern shows 6% on response to Covid-19 vaccine in older adults; Analyst says Buy Now AstraZeneca Rises in Trump Report Could Accelerate Vaccine Candidate Covid-19 Abbott by expanding its Covid-19 test to asymptomatic people – Report
Buffett Berkshire has built positions in Japan’s five largest trading companies
Goldman increases Peloton PT in 4Q growth potential
At
Merck vs. Merck Eli Lilly: what pharmaceutical inventory is an option?