“o. itemList. length” “this. config. text. ariaShown”
“This. config. text. ariaFermé”
Merck
The Phase 1/2 trial, which will take place in Belgium with 260 registered subjects, was reported through Merck in a government database and shown through the company, the Wall Street Journal reports.
According to the publisher, Merck stated that the dose in the subjects examined had begun, but provided additional comments.
Merck secured the rights to V591 through the acquisition in May of Themis, an Austrian vaccine manufacturer that presents vaccines and treatments on a cutting-edge measles virus vector platform.
The “measles vector is designed to provide a vehicle to administer antigens to the immune formula capable of triggering a protective reminiscence response,” corporations said at the time.
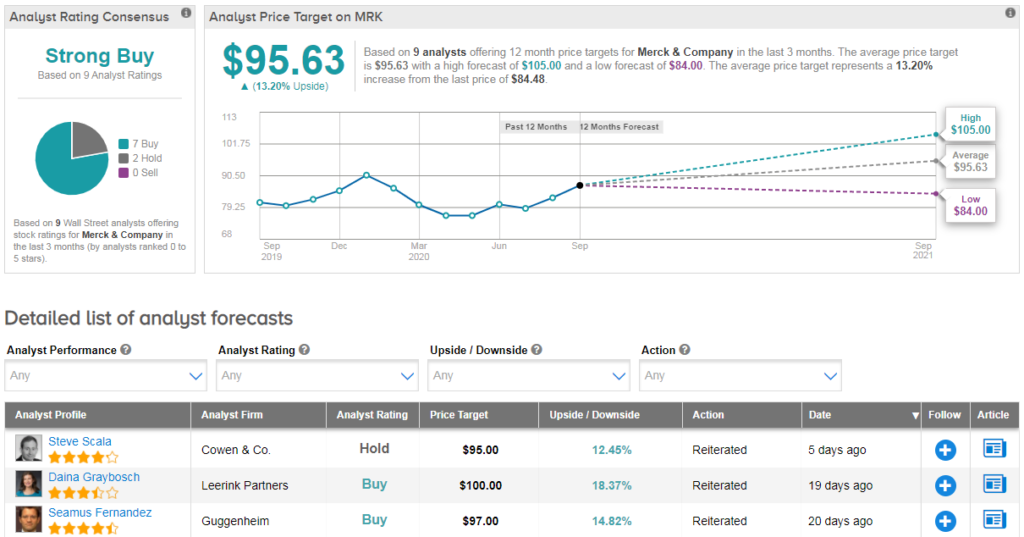
Merck’s shares are trading down 7% since the beginning of the year, however, analysts have a strong Strong Buy consensus on stocks, which is divided into 7 acquisition notes instead of 2 maintenance notes. $96 implies a 13% forward-looking build-up for next year.
Mizuho Securities analyst Mara Goldstein recently reiterated a purchase note to rent in inventory with a $100 value target (reflecting a bullish potential of 18%), saying oncology is recovering from Covid-19.
“Keytruda’s expansion continues to drive the transformation of
Related news: Novavax publishes 8% of Covid-19 vaccine data; An analyst says he’s buying Medtronic’s first formula for youth diabetes approved through Gilead Sciences for about $20 billion in immunomedia ink – Report
JetBlue 24 new routes to capture traffic
Atara Bio rises after sclerosis drug shows stable improvement
Tesla in talks with Giga Metals of Canada for low carbon nickel: report
UBS reduces Expedia’s score to deal with concern calls; Sharing autumn