“o. itemList. length” “this. config. text. ariaShown”
” this. config. text. ariaClosed “
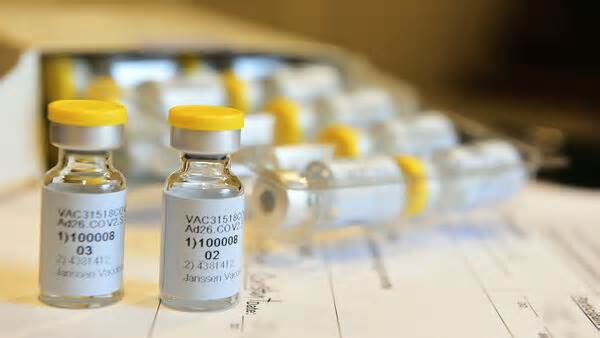
First participants treated in the Phase 3 trial (ENSEMBLE) comparing the protection and efficacy of the Janssen COVID-19 candidate vaccine, JNJ-78436735, known as Ad26. V2. S
NEW BRUNSWICK, New Jersey, 23 September 2020 / PRNewswire / – Johnson
Johnson
Johnson
“While COVID-19 continues to have an effect on the daily lives of others around the world, our purpose remains the same: to leverage our company’s global success and clinical innovation to help end this pandemic,” said Alex Gorsky, President and CEO, Johnson
“We remain fully focused on finding an effective COVID-19 vaccine that is urgently needed for others around the world,” said Paul Stoffels, M. D. , Vice President of the Executive Committee and Chief Scientific Officer, Johnson
The Janssen COVID-19 candidate vaccine is located on the company’s AdVac® generation platform, which has also been used to expand and manufacture the Ebola vaccine approved through Janssen’s European Commission and create its candidates for Zika, RSV and HIV vaccines. The platform has been used to vaccinate more than 100,000 people to date as a component of Janssen’s experimental vaccine programs.
With Janssen’s AdVac® technology, it is estimated that the vaccine, if successful, at the time of release will remain solid for two years at -20 degrees Celsius and at least 3 months at 2-8 degrees Celsius, making the candidate vaccine compatible with the most popular vaccine management channels. and would not require new infrastructure to deliver to those who wish.
PHASE STUDY TOGETHER
The Phase 3 ENSEMBLE exam is a randomized, double-blind, placebo-controlled clinical trial designed to evaluate the protection and efficacy of a single placebo vaccine dose in up to 60,000 adults 18 years of age or older, adding significant representation of the over-60s The trial will come with others with and without comobility related to a greater threat of progression to COVID-19 severe , and will aim to recruit participants in Argentina, Brazil, Chile, Colombia, Mexico, Peru, South Africa and South Africa. United States. In order to compare the effectiveness of Janssen’s COVID-19 vaccine, countries and clinical trial sites that have the highest incidence of COVID-19 and the ability to achieve rapid commissioning will be activated.
Based on a legacy of objective-based movements and a commitment to diversity and inclusion, the Company aims to represent populations that have been disproportionately affected by the pandemic in the implementation of its PHASE 3 COVID-19 test program. this includes a significant representation of black, Hispanic/Latino, Native American, and Alaska Native participants.
ENSEMBLE is presented in collaboration with the Advanced Biomedical Research and Development Authority (BARDA), which is a component of the U. S. Office of the Undersecretary for Preparation and Response to the U. S. Health and Human Services Decomposant (HHS)But it’s not the first time Under transaction agreement HHSO100201700018C, and the National Institute of Allergy and Infectious Diseases (NIAID), a component of hhS’s National Institutes of Health (NIH).
At the same time, the Society previously agreed to work with the United Kingdom of Great Britain and Northern Ireland (the UK government) on a separate Phase 3 clinical trial in several countries to explore a two-dose regimen of the Janssen candidate vaccine.
“With our candidate vaccine now in our global Phase 3 trial, we are about to find a solution for COVID-19. We use a highly clinical and evidence-based technique for this candidate vaccine. We are incredibly grateful for the tireless efforts of our researchers and for the important contributions of participants who volunteered to participate in our studies. Together, we are running to help combat this pandemic,” said Mathai Mammen, MD, Ph. D. , Global Head, Janssen Research
The Company is conducting ongoing discussions with many stakeholders, adding national governments and global organizations, as a component of its efforts to meet its commitment to making the candidate vaccine available worldwide, provided that the vaccine is shown to be effective and after regulatory approval.
To learn more about Johnson’s multiple approach
About Johnson
In companies like Janssen Chez Janssen pharmaceutical companies, we are creating a long-term where the disease belongs to the past. We’re Johnson’s pharmaceutical corporations.
Notice to investors about forward-looking statements
This press release “forward-looking statements” as explained in the Private Securities Litigation Reform Act of 1995 regarding the progression of a possible preventive vaccine opposed to COVID-19. Readers are cautioned not to rely on such forward-looking statements. If the underlying assumptions turn out to be erroneous or known or unknown hazards or uncertainties materialize, the actual effects may differ materially from the expectations and projections of Janssen and/or Johnson pharmaceutical corporations.
Logo: https://mma. prnewswire. com/media/403394/Johnson_and_Johnson_Logo. jpg
FUENTE Johnson