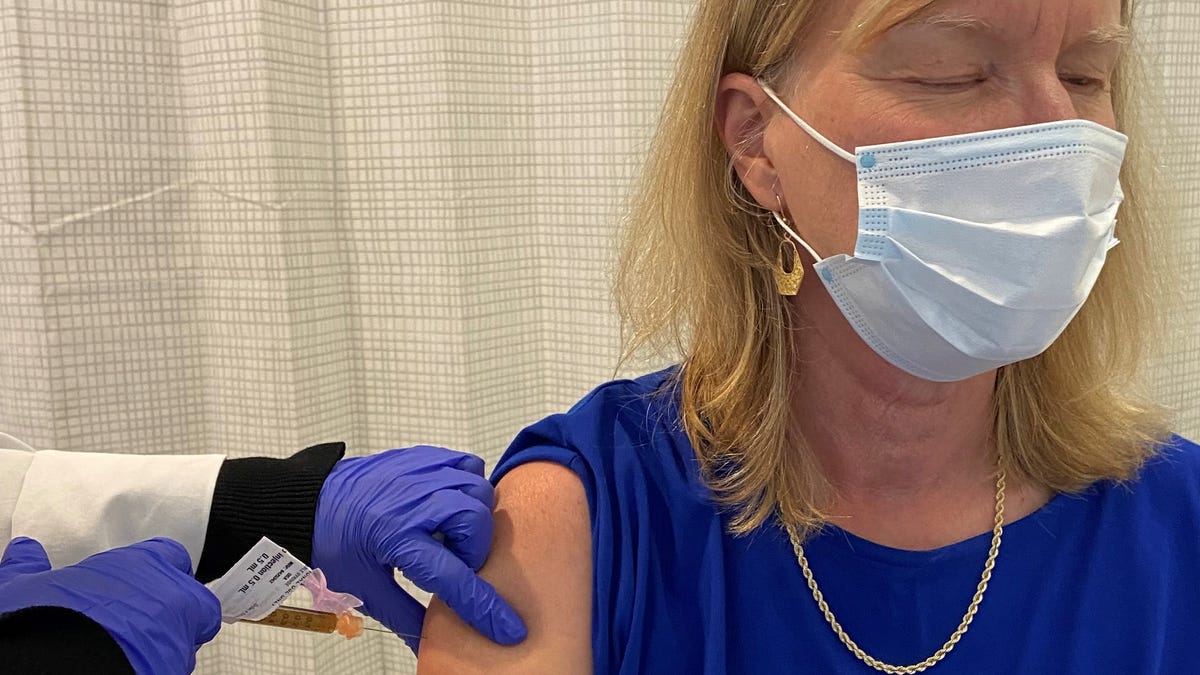
On the afternoon of September 22, I made a point of knowledge in the search for a vaccine to save him COVID-19.
That’s when I won the first of two vaccines in a clinical trial to expand a vaccine, and I was one of 30,000 volunteers to take a needle for science.
Why am I doing this? A mixture of altruism, interest and a sense of duty as a journalist, but about that later.
Apart from the nurse who injected me and the hospital pharmacy that gave you the injection, no one else knows if I won a placebo or the possible vaccine. Not me. Not even Dr. Bindu Balani, the lead researcher in the trial at Hackensack University Medical Center, one of 89 sites across the country.
This is called a double-blind test because researchers and participants don’t know what’s inside that syringe.
I confess, I have a hunch. But I probably don’t percentage it, in case the team watching me reads it.
The proven vaccine was developed as a component of THE’s Warp Speed operation. But it’s not the first time Through ModernaTX, a biotechnology company in Cambridge, Massachusetts. Moderna earned $955 million in government investment for the project, never brought a vaccine to market. If this vaccine is safe and effective, the federal government is committed to purchasing one hundred million doses, with an option of 400 million more.
Seven days after the injection, I took the temperature every night, measured the length of the hump, the length of a mosquito bite on my arm when it disappeared, and noticed that at first my arm hurt a little, but “there are not enough daily activities. “I recorded this and other data, adding my lack of headaches, fatigue, muscle pain and nausea, in a safe phone app that sends knowledge to Moderna.
My adventure in the curtain booth where I won the first injection started at work. I’m a fitness reporter who’s been covering the pandemic for six months when I wrote an article about clinical trials of the vaccine in New Jersey.
I wanted to do anything to help and I was fascinated by how a vaccine can evolve and advertise so temporarily in the middle of a pandemic. I think a first-person account of what it’s like to be a guinea pig in those days makes a smart story.
So I finished an online questionnaire pointing out my interest in volunteering. A few weeks later, a nurse tracked a phone call.
His contagious enthusiasm. She and other nurses had volunteered to paint weekends to recruit volunteers, she said. I was extremely happy to be part of a task to end the pandemic.
The odds were 50-50 that he would get a placebo, he knew that, so there would be no harm. If I was given a vaccine and it passed, I advanced in the game. But if they gave me the vaccine and they didn’t approve, it got me thinking.
These were my doubts: after receiving a vaccine that did not make the cut, could I still get an approved vaccine or could it interact harmfully in my body?Did I potentially gain an exaggerated reaction in my immune formula and aggravate a potentially benign case?
When I told the nurse I had questions, Dr. Balani answered me.
There’s not enough knowledge to answer those questions, he tells me. I lost retiring at any time before the scheduled exam to finish in 25 months. If any other vaccine was approved and I sought to get it, she would ask for it “to eliminate ” my condition. If I got sick, they’d touch my doctors.
There were other points to consider as well. As a fitness writer, I’ve been reporting for months on the devastation caused by the coronavirus in New Jersey, from the rush through hospital beds to the incalculable loss of human life. I have witnessed more pain and worry in seven months than in my last 40 years. Participation in the search for a vaccine felt like a small act of defiance and an expression of non-public hope.
In addition, I was interested in science and its translation into public health: the frantic pace, the advances in understanding, the enormous scale of logistics to produce and distribute vaccines to an entire nation, not to mention the world.
It sounds historic, like the Manhattan Project, possibly the last time the United States government, science and industry worked in combination on such a scale. I inherited the feeling that science can replace the globals of my father, who worked as a nuclear scientist in Hanford, the former outpost of the Manhattan Project in Washington state.
Me to go.
On the day of my appointment, I read and signed the 22-page consent form. Balani took my medical history, did a physical exam and rubbed me to see if I had an active COVID-19 infection. A nurse took my important symptoms and 8 vials of blood. I downloaded the app and learned how to use it.
The injection into my right arm is quick and easy.
I had a statistic.
Moderna’s vaccine is in a new approach to generating an immune reaction in the receptor: it uses messenger RNA (mRNA), a component of cells that transmits genetic information, to induce the framework to produce coronavirus antigens and thus stimulate the immune system. vaccines for other diseases, does not use total, live or inactivated viruses.
The first tests of the Moderna vaccine in humans (Phase 1) had forty-five participants; Phase 2 had 600; I’m in phase 3.
Phase 1 receptors have developed an immune reaction opposed to the disease, many antibodies, according to a report published in the New England Journal of Medicine.
Three dose sizes were tested to determine which was best. Three of the other 14 people who won the maximum dose of the vaccine reported serious side effects after the dose at the time, adding a type who had a high fever, vomited and fainted the next day.
This dose is 21. 2 times the dose my control organization received. It was established at a decreasing and more tolerable point for phase 3.
Moderna did not imply whether COVID had evolved after receiving the vaccine.
This question, if the vaccine prevents your receptors from appearing COVID-19, it will have to be resolved through the Phase 3 trial, which began at the end of July, and we, as volunteers, will have to get two injections of one hundred milligrams each, 28 days apart. Half of us get a placebo, salt water, and the other part, the genuine.
To date, Moderna has recruited 28,043 participants. More than 19,000 have already won their doses.
They will follow us to see if we expand COVID-19 and, if we do, how seriously we are sick and whether we want to be hospitalized.
As soon as 53 other people get a proven diagnosis of COVID-19, proponents, and an independent external advisory organization called the Data And Security Oversight Committee, will make the first mid-term evaluation to compare the number of other people with and without the vaccine. he was infected. Another assessment is planned after 105 instances among participants, and the final assessment will have to be performed after 151 instances. Security surveillance will continue for some time after that.
The vaccine will be considered effective if it reduces the threat of COVID by 60%, i. e. if it prevents six out of 10 recipients from having COVID-19 (the flu vaccine is 40% to 60% effective). ) Modern would possibly ask the Federal Food and Drug Administration for emergency use authorization, or the U. S. , if there is strong evidence of efficacy and protection after the first mid-term evaluation.
Moderna CEO Stéphane Bancel said on October 1 that the company can apply for an U. S. until November 25, assuming safety knowledge is good.
An emergency use authorization is more flexible than full FDA approval of a vaccine. The last few months have shown how it can be politicized. At the beginning of the pandemic, after President Trump promoted the use of hydroxychloroquine to treat COVID-19 patients, he won an OAS and then canceled when studies showed it was useless and could in some cases cause harm.
The president also announced a U. S. for convalescence plasma, the antibody-rich serum extracted from the blood of COVID-19 survivors on the eve of the Republican National Convention, based on knowledge that some experts are unstable.
Full approval of the vaccine, which requires long-term protection control, takes years.
The Moderna is designed to last 25 months. After taking my moment, I will make 4 more visits to the clinic, enter my knowledge in the phone app at other times and get regular follow-up phone calls. Researchers will monitor us for side effects and destructive effects for two years.
Modern and Pfizer released their detailed examination protocols in September. The obvious objective was to reassure the public about the clinical integrity of the procedure without regard to considerations of political pressure.
To one question, I get a lot: no one will intentionally disclose the virus to me in this study.
A top component threat is not a component of the agreement. I do not intend to walk unsyged in an extensive care unit for COVID patients. To be honest, I don’t even plan to dine inside a restaurant.
This warning, which experts say is shared through other volunteers in the trial, would possibly slow down the search; The faster the instances accumulate, the faster experts can see if the vaccine is working.
Unfortunately for science studies, other people who volunteer for studies like this tend to be more informed and more health-oriented, said Dr. William Schaffner, professor of medicine at Vanderbilt University Medical Center and a member of the Center’s Advisory Committee on Immunization Practices. prevention and disease.
As a result, they are more likely to be alone by COVID-19, which means it will be some time before a sufficient number of infections accumulate.
Since the polio epidemic, the public has not been more obsessed with the vaccine race Who will get the first batches?How will it be distributed?
All those unanswered questions. But I feel like I’m part of the story now.
I’ll let you know what’s going on.
Follow Lindy Washburn on Twitter: @lindywa