Supports nonprofit journalism.
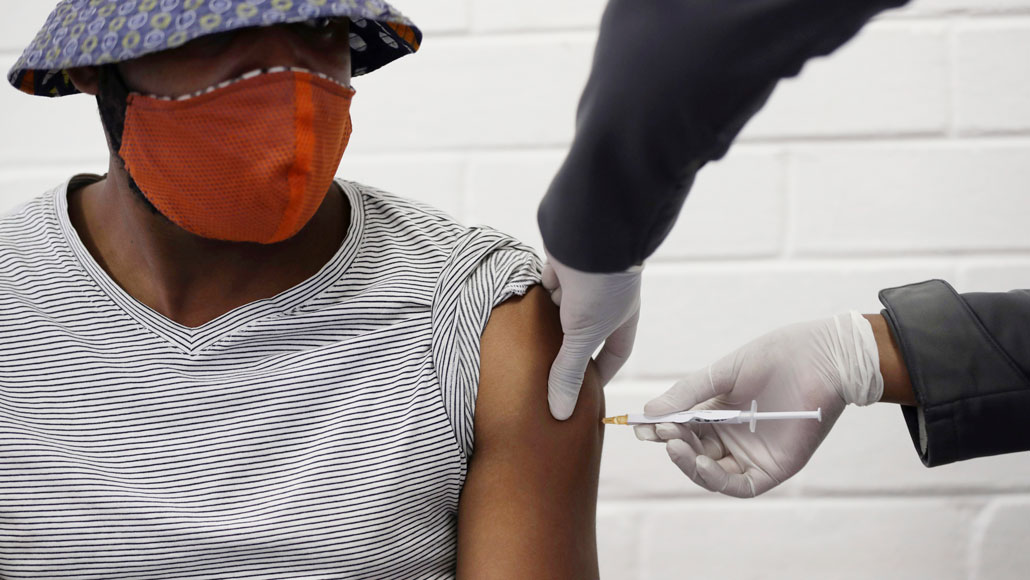
The pharmaceutical company AstraZeneca has suspended all clinical trials of its coronavirus vaccine due to a possible adverse reaction in a volunteer. The pause, which affects ongoing trials around the world (one volunteer was vaccinated in the trial in South Africa), will allow an independent panel to determine whether the disease originated from the vaccine or was a coincidence.
Photo AP / Siphiwe Sibeko
The illness of an unmarried volunteer has brought a complex clinical trial of a leading vaccine against coronavirus to a transient halt, an action that underscores the point of rigor needed to make sure a vaccine is effective, experts say.
AstraZeneca, who is preparing the vaccine in collaboration with Oxford University, took a break on 8 September after an examination volunteer in the UK had a suspected serious reaction. The pause will allow a separate review panel to do next.
The disease may have nothing to do with the vaccine. If so, the test can be resumed, which can recruit up to 50,000 people worldwide, adding up to 30,000 in the United States. If the vaccine causes illness, known as a serious adverse event, it may also spell the end of hopes for the AstraZeneca vaccine. But experts say the breakdown is part of the delicate task of science and will have to happen to ensure some security.
“It was heartening to see AstraZeneca take it so seriously,” says Esther Krofah, executive director of FasterCures, a Washington, D. C. -based nonprofit that is part of the Milken Institute think tank. “They did precisely the right thing. “
AstraZeneca is among the pharmaceutical corporations testing COVID-19 vaccines that, in an open letter published Sept. 8, pledged not to be rushed by political considerations and to stick to popular procedures so that vaccines are thoroughly tested.
What the public sometimes doesn’t realize is that clinical trial courses sometimes don’t go well and phase III trials are temporarily suspended, says Seema K. Shah, a bioethicist at Lurie Children’s Hospital in Chicapass. In fact, “hurdles in it are general to vaccine trials, and they deserve to happen if you look at them ripassively,” he says. “If nothing wrong happens while you’re testing it, you may not have tested it well enough. ” .
We’ll have to wait to see if there’s a security factor or if it was a false alarm, he said. “Normally that would take place and would not be foreign news. But right now, everyone is watching those vaccine trials and we’re all holding our breath while we wait for the results. “Science News spoke to experts about what a long-term rupture of a coronavirus vaccine might mean.
Titles and summaries of Science News articles, sent to your inbox
The vaccine is a mixture of two viruses. Researchers from Oxford and a spin-off from Vaccitech University started with a weakened edition of an adenovirus that causes colds in chimpanzees, the same chimpanzee adenovirus that is used to make an Ebola vaccine. make the iconic knobby protein “spike” of SARS-CoV-2, the virus that causes COVID-19 (SN: 7/21/20).
Some other possible coronavirus vaccines that are being tested lately use human adenovirus to send the peak protein. But because many others have had colds caused by adenovirus, they may already have antibodies that can also make the vaccine less effective. Using a chimpanzee virus that doesn’t infect other people can also solve this problem.
In preclinical testing with rhesus macaques, the vaccine that fights against coronavirus infections, the researchers reported July 30 in Nature. And in early human studies, the vaccine stimulated the production of antibodies opposed to the spike protein, the researchers reported online July 20 in The Lancet. This study tested the coronavirus vaccine on 534 volunteers.
These other people reported more commonly mild side effects, such as headaches, fatigue and muscle aches, but to find out if the vaccine works and is safe, you want to try it on several thousand other people. placebo-candidate vaccine. If the vaccine works, more people in the placebo organization will eventually get COVID-19 than in the vaccinated organization.
All we officially know is that one of the volunteers at him went to the hospital after having neurological problems. Some reports cited un nameless resources claiming that a woman participating in the trial had symptoms consistent with transverse myelitis, an inflammatory spinal cord syndrome.
Transverse myelitis has already appeared in vaccine trials. Symptoms range from numbness, tingling, or pain to limb paralysis and bladder problems. Doctors treat the disorder with steroids that calm the inflammatory process, although severe cases can have long-term consequences.
“In the history of vaccine development, cases of myelitis are not particularly surprising,” says Carlos Pardo-Villamizar, clinical neurologist and director of the Johns Hopkins Center for Transverse Myelitis. Although rare, transverse myelitis has made the impression in vaccine trials opposed to rabies, yellow fever and H1N1 flu, among others, he said.
The disorder is “inflammation due to an immune trigger,” it says, such as a virus, bacteria, or autoimmune disease. Rarely, vaccines can cause the same immune failure.
A similar reaction, called Guillain-Barré syndrome, related to the 1976 flu vaccine, where one in 100,000 people had a great threat of symptoms such as muscle weakness or paralysis. Since then, some vaccines have been linked to Guillain-Barré syndrome. However, this is rare. There are usually one or two cases consistent with millions of doses of vaccine, according to the Centers for Disease Control and Prevention.
“My message to the public is that I don’t panic, it’s a little expected,” Pardo-Villamizar said. These are the types of headaches that want to be rigorously evaluated before a vaccine becomes public, he says.
No, it’s a regimen if an adverse occasion is severe enough to send a user to the hospital. That’s part of the process.
One of the main problems in a clinical trial is encountering some vaccine-like fitness disorder. Some side effects are expected and manageable, such as redness or swelling at the injection site, fever, muscle or joint pain, headache, or fatigue. But serious adverse occasions want to be investigated to perceive that they were similar to a vaccine or a coincidence.
Stopping a clinical trial to investigate a serious fitness factor “is a popular practice in ongoing trials,” says Susan Ellenberg, a biostatistics at the University of Pennsylvania’s Perelman School of Medicine. Taking the time to read about an informed serious reaction is a sign that the formula is working, Says Ellenberg. “This is what is meant to happen. “
The regulations for some trials would require investigation even if a volunteer has been in a car accident, just to make sure there is no connection to participating in the trial. “These triggers are predetermined and written into protocols, so you can’t replace your mind” to bypass a potential protection issue, says Paul G. Thomas, an immunologist at St. Jude Children’s Research Hospital in MemphisArray Tennessee.
An independent knowledge security oversight committee will gather knowledge and investigate issues. These protective symptoms are required for all clinical trials. “They have no vested interest in the vaccine. They weren’t the ones who invented the vaccine. These are not other people who can make money off the vaccine, ”says Thomas.
Sometimes, tips avoid testing due to protection issues. Testing can also end if it becomes blindly obvious that one organization is doing much more than another, because one drug or vaccine works very well, Thomas says.
In the case of the AstraZeneca-Oxford vaccine, the first thing the board is likely to do is whether the woman was in the placebo organization or the organization that won the vaccine, says William Schaffner, an infectious disease physician at Vanderbilt University. Focus.
“This investigation can be very brief,” he said. “Could you locate, oh, this user had a placebo. No problem. The test can pass. It’s a coincidence.
But if the user has won the vaccine, “then we are trapped in a complicated position”. The board will want to evaluate all the data, adding the volunteer’s medical history, if the vaccine caused their illness. If the board of directors says the vaccine was the cause, “that could end with the full test of this vaccine. “Schaffner said. ” That’s how bad this occasion is and your next investigation. Very heavy. “
There is no way of knowing, from animal testing or in a smaller number of people, that such an appearance effect can occur simply when the vaccine is given to a giant number of people. Phase III trials are designed in components to discover side effects and rare reactions, Schaffner says. “This is an incredibly rare and unforeseen occasion that may not have been anticipated. “
Even if the AstraZeneca-Oxford vaccine fails, FasterCures follows 210 vaccines at other stages of development, Krofah says. “If they fail, there are many more under investigation. ” She is encouraged by the fact that the company follows the general procedures of clinical trials. “We will have to continue to focus on science and be firm about the transparency of protection and effectiveness data. “
Scientists and bloodhounds have a basic confidence in questioning, observing, and checking to be successful in the truth. Science News reports very important studies and discoveries in all clinical disciplines. We want your money to take place; each and every one of the contributions makes a difference.
N. van Doremalen et al. The ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. Posted on Jul 30, 2020. doi: 10. 1038 / s41586-020-2608-y
P. M. Folegatti et al. Safety and Immunogenicity of the ChAdOx1 nCoV-19 Vaccine Against SARS-CoV-2: An Initial Report of a 1/2 Randomized, Single-Blind Trial The Lancet. Vol. 396, August 15, 2020, p. 467. doi: 10. 1016 / S0140-6736 (20) 31604-4
Tina Hesman Saey is the lead editor and reports on molecular biology. He holds a Ph. D. in Molecular Genetics from the University of Washington at St. Louis. Louis and a Master’s degree in Scientific Journalism from Boston University.
Jonathan Lambert is the editor-in-chief of Biological Sciences, from species origin to microbial ecology. He holds a master’s degree in evolutionary biology from Cornell University.
Aimee Cunningham is the biomedical writer. He has a master’s degree in science journalism from New York University.
Science News, founded in 1921 as an independent, non-profit source for accurate data on the latest news in science, medicine, and technology. Today, our project remains the same: to train other people to evaluate existing occasions and the global that surrounds them. is published through the Society for Science
Subscribers, enter your email in the Science News file.