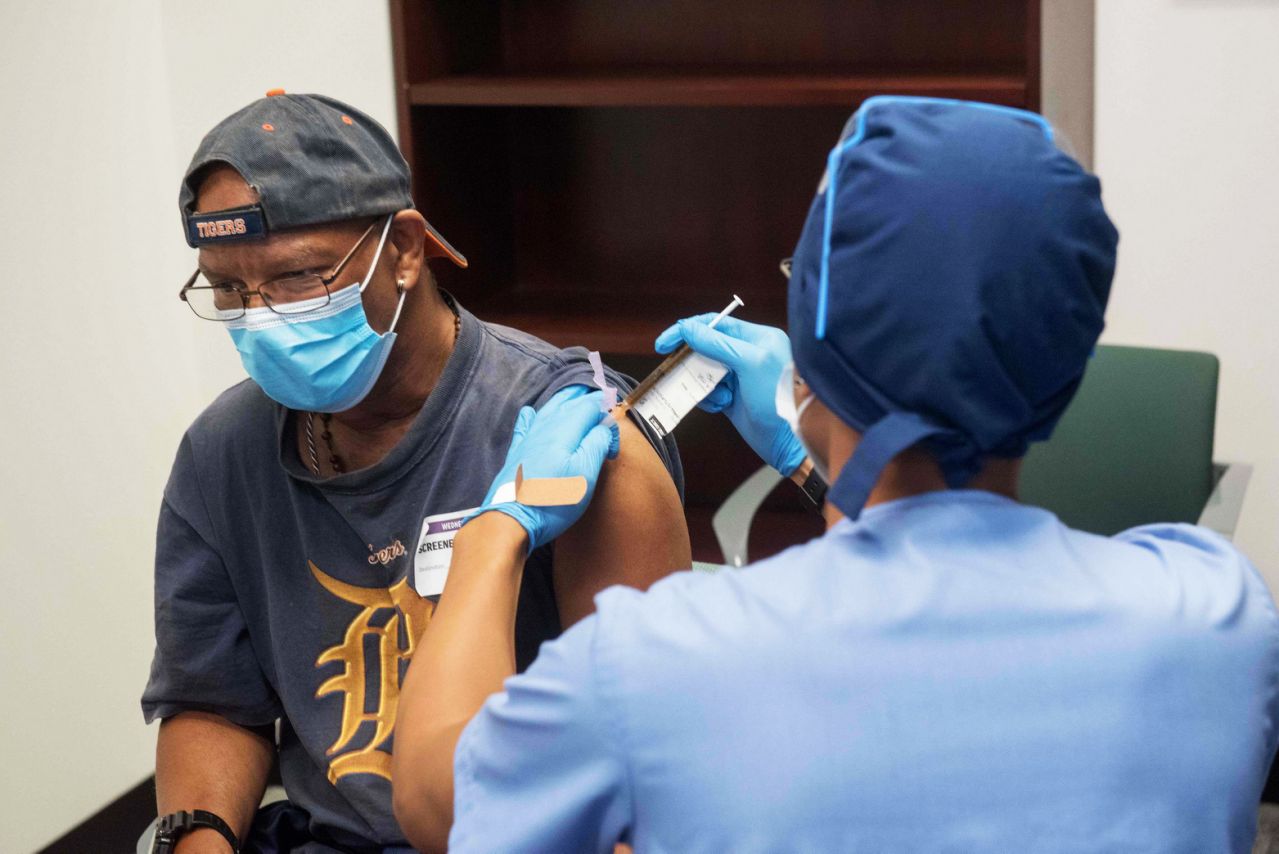
More than 30,000 volunteers have enrolled in two primary trials of the COVID-19 vaccine in the United States, he said Friday.
“Surely we’re on the right track, if not a little ahead of” the purpose of creating a safe and effective vaccine and generating tens of millions of doses by the end of the year, Paul Mango, deputy director of policy staff at the U.S. Department of Health and Human Services, said in a call with reporters.
The two vaccine manufacturers, Moderna and Pfizer, aim to recruit a total of 60,000 volunteers for their phase 3 trials. Mango said the recruiting was half-finished, but did not specify the number of other people in each trial. AstraZeneca, in partnership with the British University of Oxford, has also begun phase 3 trials of its COVID-19 vaccine, with examination sites in the United States.
Full of the coronavirus outbreak
However, “there is no guarantee in science,” Mango noted. Tested vaccines may not be effective.
President Donald Trump promised quick repaints about a vaccine in his Thursday night speech at the Republican National Convention, saying the country “will produce a vaccine before the end of the year, or perhaps even earlier.”
Officials predicted that the search would be routed even if Trump lost his re-election.
“The vast majority of people involved in Operation Warp Speed are not named by the Trump administration,” Mango said.
Download the NBC News app to complete the coronavirus outbreak
Clearly, a single vaccine will not be enough to meet expected demand. Some vaccines would possibly be more suitable for certain populations, such as the elderly or others with weakened immune systems, said Dr. Gregory Poland, an infectious disease expert who leads the Mayo Clinic’s vaccine study organization in Rochester, Minnesota.
“Young people are other adults and other older adults,” Poland said, adding that teams such as pregnant women and others with immunosuppression or autoimmune diseases may also react differently.
Phase 3 trial assistance answers these questions.
Mango said officials felt “very well” with the diversity of records. “We have a lot of other people over the age of 65. We have many other people with chronic problems. We have a Hispanic participation, we have an African-American involvement,” he said.
Half of the participants will get the vaccine and the other party will get a placebo. Who will receive which edition will be unknown to researchers and participants.
Mango predicted that more vaccines could generate larger trials, most likely until next month.
Meanwhile, Poland recalled that the cover measures shown still work: “If other people stick to the undeniable recommendation to dress in a mask, physically distance themselves and disinfect their hands, it may be so.”
Follow NBC HEALTH on Twitter and Facebook.