Eight months after the start of the COVID-19 pandemic in the United States, it is unclear whether two of the most promising remedies are working.
As a vaccine is expected until 2021, there is an urgent need for effective remedies for COVID-19. Thousands of Americans are hospitalized lately, and the Centers for Disease Control and Prevention has reported screenings of up to 205,000 deaths in the United States through mid-September.
But the clinical trials needed to provide evidence of convalescent plasma and monoclonal antibodies were delayed and had difficulty recruiting volunteers. Many trials are newborn now, months after the birth of the pandemic, as researchers have focused their initial efforts on successful therapies, such as hydroxychloroquine.
Full of the coronavirus outbreak
Randomized, placebo-controlled clinical trials, considered the gold standard, assess whether a remedy works by comparing it to placebo. Initially, neither researchers nor participants know who receives the genuine product and who gets the placebo.
This has created demanding situations for test researchers.
“There are other people who say, “I don’t need to be a guinea pig.” If you think this works, why don’t you give me the genuine? “Unlike a placebo,” said Dr. Shmuel Shoham, an associate physician in the Division of Infectious Diseases at Johns Hopkins University School of Medicine.
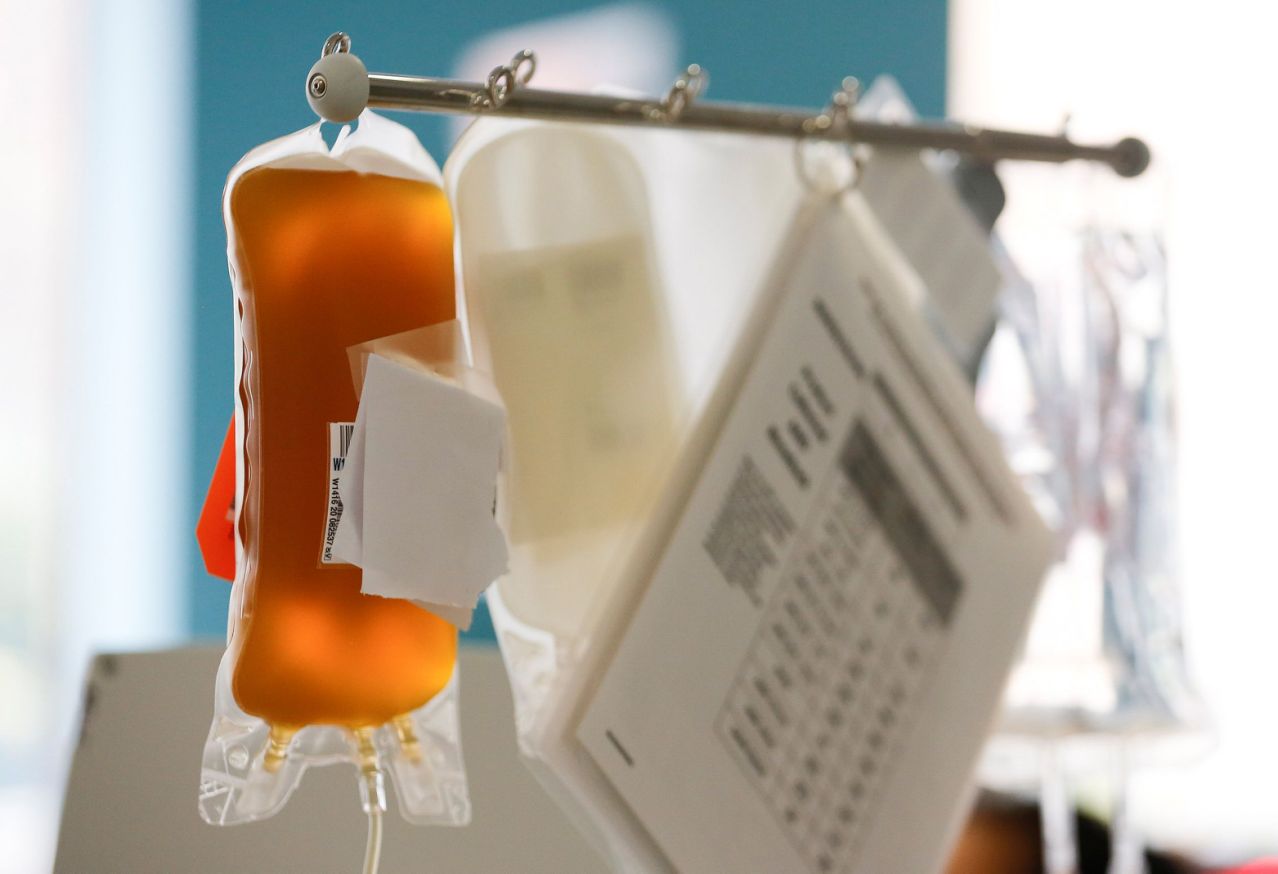
The concept of convalescent plasma and monoclonal antibodies is to provide the immune formula with a special touch to fight the virus. Convalescence plasma refers to the antibody-rich blood product extracted from patients who have already recovered, and monoclonal antibodies are an artificial edition of those antibodies that can be mass-produced in a laboratory.
But tens of thousands of COVID-19 patients who may have been eligible for clinical trials comparing those treatments have already gained convalescent plasma as a component of the expanded program running at the Mayo Clinic, leaving them out of the race to participate in the trial.
“Some of us were temporarily put on this train,” said Dr. Ashok Balasubramanyam, vice president of educational integration and senior associate dean of educational affairs at Baylor School of Medicine, bringing early evidence from China suggesting that a cure gains advantages for convalescent plasma.
“The show is very liberal,” Balasubramanyam said. “Anyone can log in and temporarily get FDA approval.”
This is not necessarily a bad thing, a pandemic in which millions of Americans have been infected. Convalescent plasma has a long history of use for other diseases and is sometimes considered safe.
While it is unlikely to hurt, and can only help, many patients decide to get plasma.
Shoham and his colleagues at Johns Hopkins are conducting a national clinical trial to determine whether convalescent plasma can save the disease or keep others healthy enough to stay out of the hospital.
But so far less than one hundred of the 1,000 planned participants have been recorded.
An exam in Florida had to close, Shoham said, because clinical trial coordinators have become too exhausted to care for COVID-19 patients. Others have struggled to locate a qualified adequate area through a blood bank to make infusions.
“We were trapped in this virus, ” said Shoham. “It took us a while to recover.”
The National Institutes of Health donated $34 million to Vanderbilt University Medical Center in Nashville for a large-scale randomized controlled trial to determine whether convalescent plasma can help very sick patients hospitalized by COVID-19.
“We were told to get this answer as temporarily as possible,” said Dr. Todd Rice, an associate professor of medicine at Vanderbilt. Up to 1,000 patients will be recruited from 50 sites across the country. Rice will lead the monumental effort.
“Plasma convalescence is not the simplest thing to obtain. It’s a blood product and, preferably, you want to pass through tests to make sure the patient has the right antibodies that will neutralize the virus,” Rice said. “It’s not like saying, “Here’s a drug.” Take. “
Federal investigators are closely following clinical trials. The U.S. Food and Drug Administration would issue an emergency use authorization for convalescent plasma, a resolution that would expand physicians’ ability to access the product.
“In accordance with the policy, we are not in a position to say whether we will take action related to the authorization of emergency use for convalescent plasma and we will make a resolution at the right time,” said Dr. Anand Shah of the FDA. Deputy Commissioner of Medical and Clinical Affairs, he said in an email.
“Science is vital because we’ve taken some bad steps in recent months,” Balasubramanyam said. The FDA had to rescind an emergency authorization for hydroxychloroquine in June after evidence showed that the drug was too complicated and did not work to treat COVID-19.
With the convalescent plasma, Balasubramanyam said: “We have a clue that it works. Now it’s incredibly safe to work.”
The effects will depend, in part, on people’s willingness to volunteer for randomized clinical trials. This means that a patient can be assigned to get a placebo, which plasma rich in genuine antibodies.
Kellie Guyton, 34, of Winfield, Alabama, knew she would want specialized care when she was hospitalized for COVID-19 in July. Previous heart surgeries and a kidney transplant have put her at high risk for coronavirus complications.
But Guyton decided to participate in Vanderbilt’s trial.
“I think if it didn’t hurt me, I totally agree, ” said Guyton. “It’s a possibility to help the next user at all times. It can just be your grandmother, your neighbor or your most productive friend.”
The source of convalescence plasma is based on enough people who have recovered from the virus to take the time to donate and is a finite resource.
This is where monoclonal antibodies can be useful. Scientists collect convalescent plasma, in the most potent and highest expression antibodies of the coronavirus, and then reproduce them in a laboratory in giant quantities.
Several drug brands expand monoclonal antibodies, adding Regeneron and Eli Lilly. Both companies began clinical trials in early June. But the effects of two of these trials, conducted through the National Institutes of Health, will not arrive until the end of this year.
Duke University’s Human Vaccine Institute pandemic rescue program recently announced that it would also expand and examine a monoclonal antibody to prevent infection with SARS-CoV-2, the virus guilty of COVID-19.
It is not known how long such coverage would last. The purpose would be to give it to other people from high-risk groups, such as those in long-term care facilities, doctors, nurses and even National Guard soldiers, if they wish in spaces with widespread infection.
Download the NBC News app to complete the coronavirus outbreak
This is because it is used in a clinic. Researchers are hoping to start recruiting patients next spring.
“We have to pass preclinical studies of protection and effectiveness,” said Grepassry Sempowski, who runs the trial. “It only takes time. We do it on a much faster schedule than we’re used to.”
All of these study projects require a judicious balance between respecting the time-consuming clinical procedure and among researchers who want to act faster than coronavirus, which has sickened nearly 6 million people in the United States. and killed more than 175,000 Americans.
“It’s just that the public perceives this clinical technique for treatment,” Balasubramanyam said. “It’s also that scientists perceive the need for speed.”
Despite the early delays, Shoham hopes that convalescent plasma will soon expand.
“In a few strong weeks we’ll be much more advanced,” he said. “Then I hope we have an answer for doctors, patients, families and the FDA.”
Follow NBC HEALTH on Twitter and Facebook.
An email leaked to U.S. Postal Service staff. He asked them not to remove mail sorting machines that had been dismantled, CNN reported. The memo, written through Kevin Couch, a maintenance operations manager, said staff “will not reconnect or reinstall machines that were disconnected in the past without HQ Maintenance’s approval.” Hours earlier, Post Office Louis DeJoy said it would be suspected that all service adjustments would end until the end of Election Day.
In a new challenge to Belarus’s authoritarian leader, many state television staff members went on strike amid an emerging wave of protests, calling for his resignation after a vote that the opposition considered rigged. This week’s hound movements have rocked the government over the media, further eroding president Alexander Lukashenko’s control after 26 years of iron rule. Vyacheslav Lomonosov, one of the Belarusian television staff members who joined the industry’s trade union action, said he and his colleagues may no longer tolerate an official ban on revealing the fact of a brutal crackdown on protests that has stoked foreign outrage.
One of the most dramatic pieces at the Democratic National Convention, in a different, heavily scripted way, was whether the new nominee, Joe Biden, would confuse his great acceptance speech with an embarrassing screw-up or an “important moment.” Well, President Trump and his crusade have been predicting this for weeks. The DNC also seemed insecure, ahead of his speech of repeated mentions of Biden’s stuttering, adding a story about his mother threatening to attack a devoted instructor who mocked his son’s stuttering elegantly and this moving video from a young Brayden Harrington.
The suspect in the beating of the driving force of a truck in downtown Portland surrendered On Friday morning, police said. “I am pleased that the suspect in this case has given up and I appreciate every effort to facilitate this safe resolution,” Portland Police Chief Chuck Lovell said Friday. A mob of troublemakers in downtown Portland hit an unconscious man Sunday night after removing him from his van, according to video footage of the incident.
Post Office Louis DeJoy said in a Senate testimony Friday that the machines will not be updated. Visit the Business Insider home page for more stories. Related Video: UsPS Fall U.S. Postal Service It will not update the mail sorting machines it has removed, Post Office Minister Louis DeJoy told the Senate Committee on Government Affairs and Homeland Security on Friday.
In a tweet, the corporation also said that 18% of participants enrolled lately are black, Latino, Native Americans or Alaskan Indians, teams that were most affected by the coronavirus pandemic. Moderna began reading its vaccine candidate, MRN-1273, in July and plans its full recruitment in September. Modern, which has never put a vaccine on the market, had in the past earned nearly $1 billion from the U.S. government, which is helping to fund several vaccine applicants through its Operation Warp Speed program.
Donald Trump’s former crusade president Steve Bannon joked about the fraud at a We Build the Wall fundraiser in a clip that resurfaced the day after his arrest. Bannon was charged Thursday along with 3 others for allegedly channeling “hundreds of thousands of dollars” from the online fundraising crusade We Build the Wall to the organization’s founder, Brian Kolfage, who among the defendants. We Build the Wall began as a GoFundMe crusade in 2018 and was created to help increase the public budget budget to move directly to the structure of the U.S.-Mexico border wall at a time when the president was suffering with Congress.
After remaining silent and unexpressed the testimony of the victims this week, DeAngelo turned to those in court on Friday, cut off his mask and rose from a wheelchair to speak, some time before Judge Michael Bowman began sentencing. “I have heard all your statements. Every one of them.
Thousands more marched through the streets of Mali’s capital on Friday to celebrate the overthrow of President Ibrahim Boubacar Keita, while the West African nation’s political opposition subsidized the army board’s plan to cede force to a transitional civilian government. Political turmoil is feared to divert attention from the more than seven-year foreign struggle that opposes Islamic extremists who have used electric vacuum cleaners in Mali to expand their territory.
Part 1 of President Trump’s interview with Sean Hannity.
Up to 215,000 more people died in the United States between January and July 2020, the Associated Press reported Friday. The number of deaths, from the U.S. Centers for Disease Control and Prevention, suggests that COVID-19 kills more people than the officially registered number. People of color make up 40% of the U.S. population, but 52% of the “excess deaths,” according to research through AP and the Marshall Project.
Donald Trump has been teased for suggesting that others needed identification to attend this year’s virtual Democratic National Convention (DNC). “To participate in the Democratic National Convention, you must have a photo ID…” In the past, those who wished to attend the DNC were asked to purchase a price ticket and present a photo ID to enter.
The Swiss electorate is expected to approve the acquisition of 6 billion Swiss francs ($6.6 billion) of new fighter jets in a referendum next month, according to a vote Thursday through the SRF broadcaster. The survey conducted through gfs.bern found that 58% of respondents were in favor of the government’s plan, 39% opposed and 3% had no opinion. European aerospace organization Airbus, France’s Dassault, Sweden’s Saab and Boeing and Lockheed Martin of the United States bid for the contract in January.
Fourteen sailors were reported to have disappeared friday after two shipments collided in the Yellow Sea east of Shanghai, the Chinese government said. Three other members of the team were rescued from the water after Thursday’s sunrise collision between a shipment carrying 3,000 tons of gas and another loaded with gravel, according to the Shanghai Maritime Safety Authority. The collision occurred outdoors at the mouth of the Yangtze River, a busy shipping lane.
Just because you want them doesn’t mean you can’t be wonderful. It originally appeared in Architectural Digest
Southwest Airlines will reject a $2.8 billion loan from the federal government through the CARES Act. Control of the airline believes it has sufficient liquidity in the form of liquidity and short-term investments, as well as possible long-term access to more financing, to help it succeed in the COVID-19 crisis, according to an investor update filed with the SEC. Wednesday. Southwest grossed approximately $18.7 billion net in 2020 and lately has approximately $15.2 billion in cash.
Donald Trump Mail in Getty Images/Exhibit President Donald Trump, obsessed with the concept that mail voting inspires voter fraud, and his crusade has sued Pennsylvania and other states for their plans to inspire the electorate to use mail-in ballots in November. Choice. Journalist Richard Salame, in The Intercept, reports that in reaction to the Pennsylvania trial, Trump’s crusade asked to show evidence that the mailing vote inspires voter fraud, and he was unable to.
The alleged $20 counterfeit bill used through George Floyd was not inspected or recovered before his fatal arrest in Minneapolis, according to one of the first officials at the scene. Former police officer Thomas Lane said in a new audio that he did not receive the bill before or after the incident that led to Floyd’s death on May 25. In reaction to questions from the Minnesota Bureau of Criminal Learning, Mr. Lane stated that he had not proven the alleged $20 forgery retained through the Cup Foods employee who identified Mr. Floyd.
The Oklahoma Zoo featured in the Netflix documentary “Tiger King” closed its doors after the federal government investigated him for alleged animal abuse and suspended his license. owner Jeff Lowe for 21 days. The zoo, in the past through Joseph Maldonado-Passage, also known as Joe Exotic, has become famous after appearing in Netflix’s “Tiger King: Murder, Mayhem and Madness”.
Peter Marks, director of the Food and Drug Administration’s Center for Biological Research and Evaluation, reacted to the considerations raised in a convention call over last week by government officials, pharmaceutical executives and academics on an organization that administers vaccines organized through the National Institutes. Health. Fixed according to 3 resources near the topic. Scientists, public fitness officials, and lawmakers are involved in the Trump administration lobbying the FDA to authorize a COVID-19 vaccine before the November presidential election, even though knowledge of clinical trials does not help their widespread use. Marks told Reuters that he had not been subjected to any political tension and that the FDA would be guided solely by science.