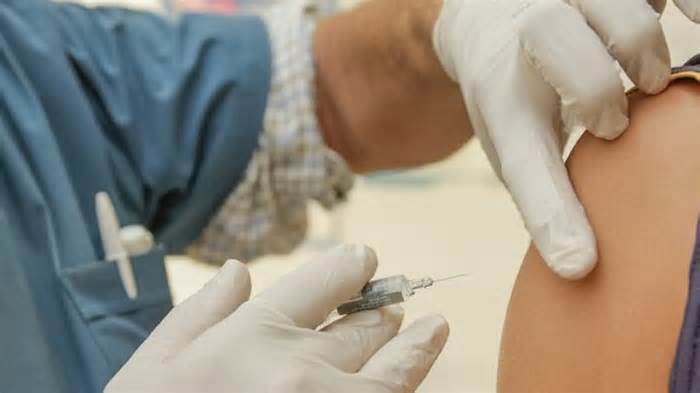
In a first step towards its fight against COVID-19, the government approved the emergency use of an Omicron-specific mRNA-based booster vaccine called GEMCOVAC-OM, evolved indigenous platform technology,® the first vaccine of its kind manufactured in the country.
The revolutionary vaccine uses a robust mRNA platform, similar to the prototype vaccine that evolved against the Wuhan spot. What sets it apart, however, is its ability to generate a physically powerful immune reaction, particularly targeting the Omicron variant. This is a step in the fight against the evolving nature of the virus, thus safeguarding public health.
The Department of Biotechnology (DBT) has announced that India’s General Drug Control (DCGI) has given the green signal to the vaccine that evolved through the national company founded in Pune Gennova Biopharmaceuticals, supported by the COVID Suraksha project and implemented through the Biotechnology Industry. Research Assistance Consultancy (BIRAC).
A notable feature of this vaccine is its heat-stable nature, it does not require an ultra-cold chain infrastructure for storage and distribution. Unlike other mRNA-based vaccines, GEMCOVAC-OM® can be stored and transported at temperatures ranging from 2 to 8 degrees Celsius, leveraging existing infrastructure and making it easily deployable across the country.
Rs 2000 for two doses: value of cervical cancer vaccine Ceravac in India
In booster clinical trials, this vaccine has demonstrated particularly superior immune responses when administered intradermally in a needle-free injection device. The clinical result demonstrates the need for vaccines with specific variants for the desired immune response.
DBT facilitated the status quo of Gennova’s next-generation mRNA-based vaccine production to expand the generation from proof-of-concept platform to Phase I clinical trials. Vaccine Development Mission” of the DBT committed allocation implementation unit at BIRAC, for the clinical progression and scaling of the vaccine prototype, according to an official version.
Commenting on the development, Gennova’s Chief Executive Officer, Dr. Sanjay Singh, said: “Obtaining GEMCOVAC-OM’s® DCGI clearance is a testament to our efforts to launch, maintain and activate this ‘pandemic-ready’ technology. India has now evolved not one but two This immediate, disease-independent vaccine platform technology with COVID-19 mRNA. »
Commending the efforts of the DBT team, Union Minister of Science and Technology Dr Jitendra Singh said, “Once again, DBT has fulfilled its mission: to enable generation-driven entrepreneurship by creating this generation of indigenous mRNA platform. We have supported generation-driven innovation in creating a “future-proof” generation platform.
“The infrastructure exists to deploy the vaccine in India, adding ICL, at 2-8 degrees C and this innovation fits into the existing supply chain infrastructure. The vaccine doesn’t want ultra-low temperature situations for shipping and storage,” he said. . Aggregate.
DBT Secretary and BIRAC President Dr. Rajesh Gokhale said strategic budget injection is key to stimulating and creating an ecosystem for technological innovation, and this is precisely what DBT did when it supported the progression of the country’s first mRNA-based platform technology.
It is a disease-independent platform and can be used to manufacture other vaccines in a short progression time. Clinical trial networks with hospital consortia were supported through NBM-DBT and the same sites were used for clinical trials of mRNA vaccines, he added.
‘título_tab’
‘adgTitle’