WASHINGTON – The federal government on Wednesday presented an ambitious plan to get COVID-19 vaccines released from the rate of all Americans, even when polls show a strong existence of skepticism spreading across the country.
In a report to Congress and an attached “playbook” for states and localities, federal fitness agencies and the Department of Defense defined complex plans for a vaccination crusade that would begin gradually in January or perhaps later this year, in the end getting any Americans who the Pentagon is concerned about the distribution of vaccines , however, civilian fitness personnel will be the one who will donate the vaccines.
The crusade is “much broader in scope and complexity than seasonal influenza or responses to vaccines similar to a previous outbreak,” says the Centers for Disease Control and Prevention’s State Handbook.
Among the highlights:
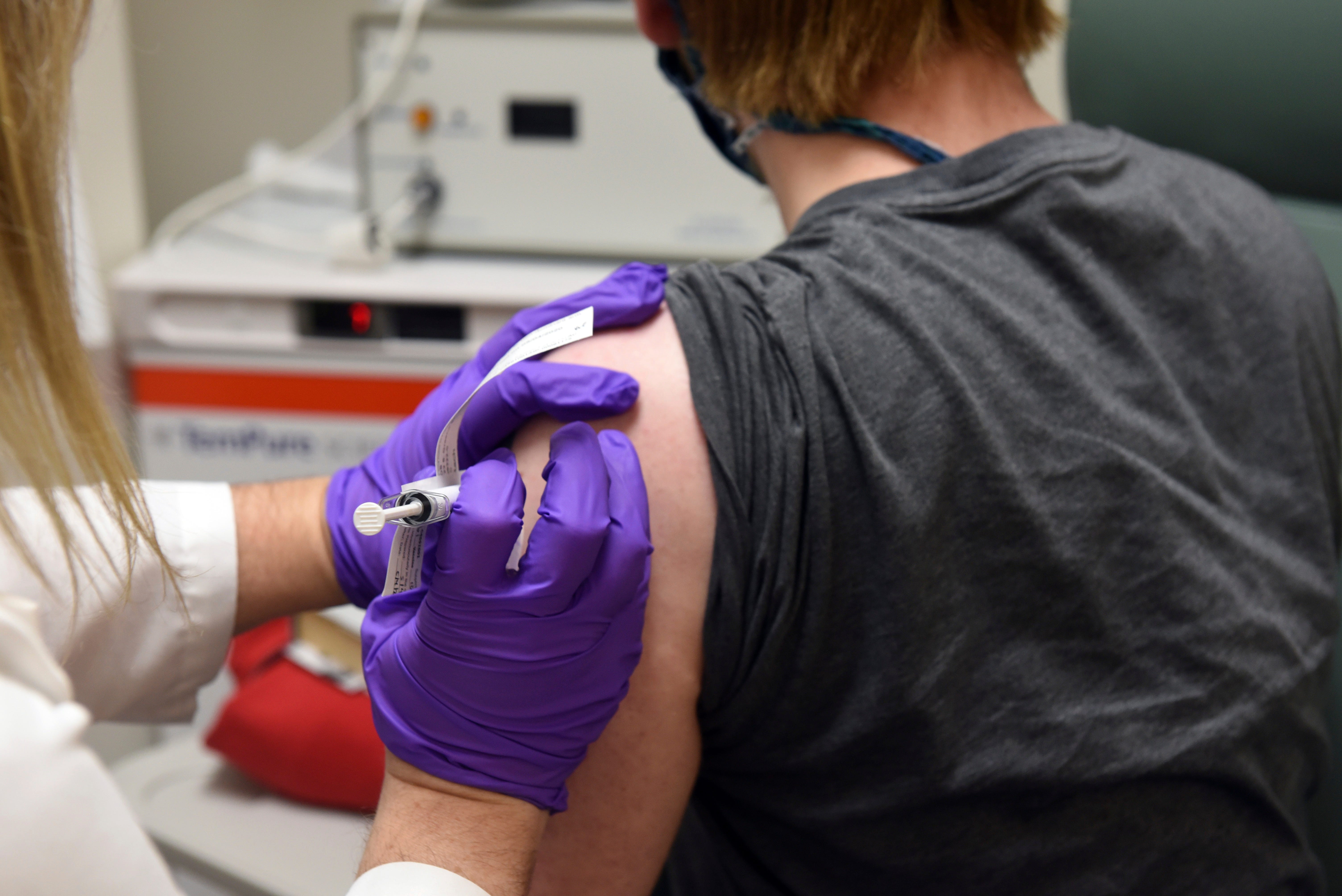
– To get the most from vaccines, other people will want two doses, spaced 21 to 28 days apart. Double-dose vaccines come from the same drug manufacturer. There may be several other brand-approved vaccines available.
– Vaccination of the American population will not be a race, but a marathon. Initially, the source of available vaccines may be limited and the concentrate will be on protective fitness workers, other essential workers and others from vulnerable groups. The National Academy of Medicine is being implemented in the priorities of the first phase. One moment and the third phase would do more vaccination across the country.
– The vaccine itself will be free and patients will not be charged out-of-pocket by the vaccine administration, thanks to the billions of dollars in public investment approved by Congress and allocated through the Trump administration.
– States and local communities will want to expand express plans to obtain and distribute vaccines locally, some of which will require special manipulations such as refrigeration or freezing. States and municipalities have one month to submit their plans.
Latest vaccine news: Pfizer ‘potential’ with COVID-19 candidate vaccine after extending the trial to 44,000 people
Some of the key parts of the federal plan have already been discussed, but Wednesday’s reports try to put key main points in a comprehensive framework. Distribution is under the auspices of Operation Warp Speed, a White House-backed initiative to make sure millions of doses are able to be sent once a vaccine is given, which is an emergency use approval through the Food and Drug Administration.
Several formulations are being finalized.
But the whole company faces public skepticism. Only a portion of Americans said they would receive the vaccine on an Associated Press ballot in May. Of those who did not receive the vaccine, the overwhelming majority said they were involved with safety. To protect the country from coronavirus, experts say more than 70% of Americans want to get vaccinated or have their own Immunity against COVID-19.
Since the poll, questions have multiplied as to whether the government is seeking to accelerate remedies and vaccines that oppose COVID-19 to President Donald Trump’s re-election possibilities.
Prior to the Republican National Convention in August, the FDA legalized the remedy of patients with COVID-19 with plasma from others who recovered, even though some government scientists were not convinced that clinical evidence was strong enough. And last week, it was, reported that Michael Caputo, a policy officer in the Department of Health and Human Services, had tried to take editorial control of a weekly clinical publication through the Centers for Disease Control and Prevention.
Timeline: The script for creating a COVID-19 vaccine began in January. The final line awaits you.
While public confidence in major fitness agencies has been defeated, Trump’s leadership has been forced to act in defense.
“We work a lot with our state and our local public fitness partners Array. . . to make sure Americans can get the vaccine as temporarily as you can imagine and vaccinate with confidence,” HHS Secretary Alex Azar said Wednesday. “Americans want to know that the vaccine progresses the procedure is based entirely on science and data. “
This can be a complicated sale. In the AP survey, 1 in five Americans said they would receive a coronavirus vaccine and 31% said they were safe.