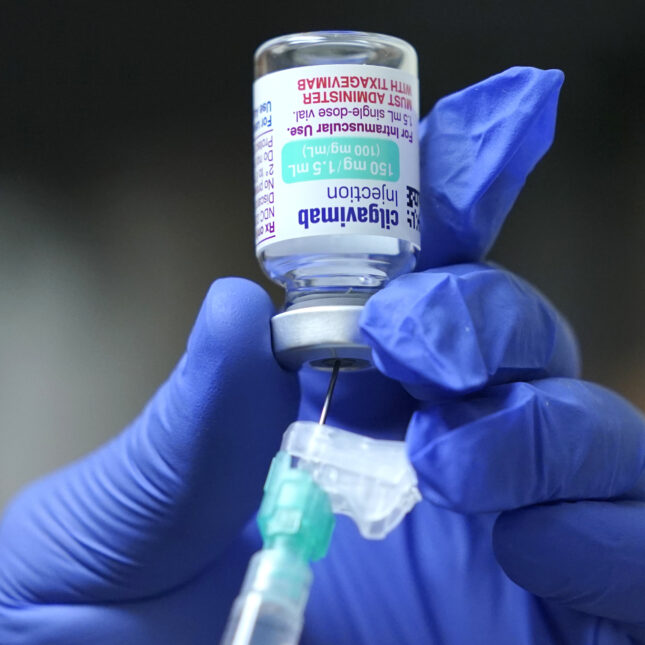
The FDA’s announcement Thursday indicated that Evusheld lost its authorization because sublines that are not neutralized by treatment are to blame for at least 90 percent of infections today. look forward to the future.
advertising
In a statement, AstraZeneca said it has begun testing another antibody that, in laboratory studies so far, has been able to neutralize all variants. The therapy, which would also be given as pre-exposure prophylaxis to immunocompromised people, could be available later this year if the trials are successful, the company said.
The company also noted that Evusheld remains authorized in other countries, adding the European Union and Japan.
Get your daily dose of exercise and medicine every day of the week with the STAT Morning Rounds newsletter. Register here.
coronavirus
drugs
public health
Report on the frontiers of medicine and
Unlimited to essential journalism in biotechnology, medicine and life sciences
Unlimited to the healthcare news and data you need