AARP Rewards makes your next steps easy, rewarding and fun! Learn even more.
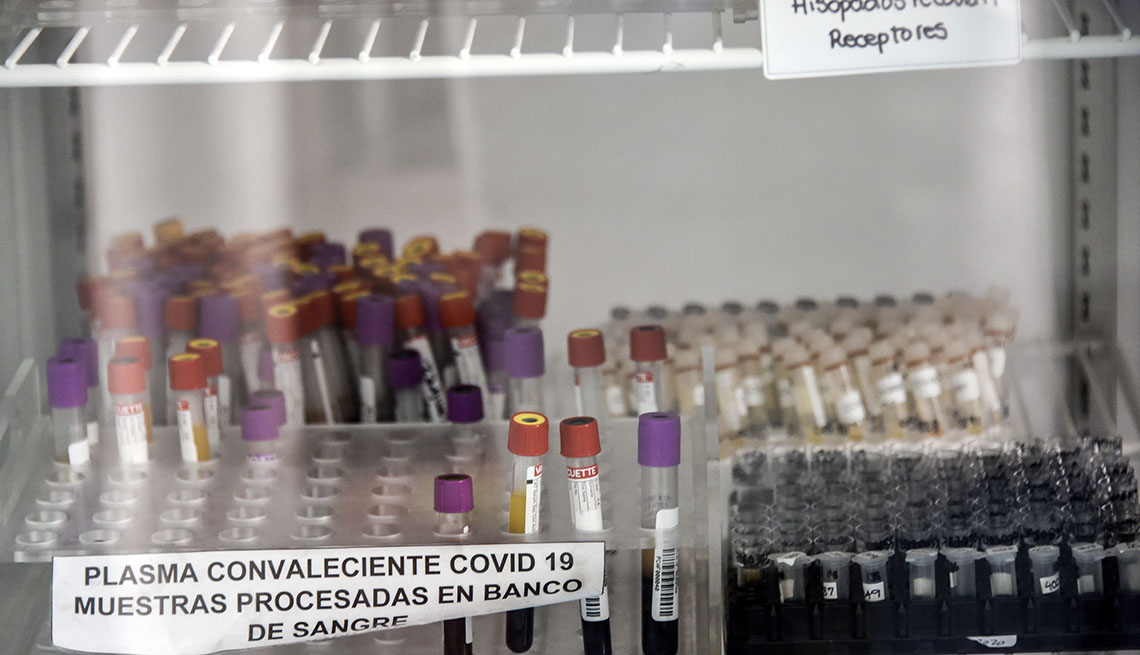
For months, the public and scientists have had high hopes of using so-called “convalescence plasma healing,” which involves an injection of the liquid component of the blood comprising antibodies from those who have recovered from coronavirus, to strengthen the body’s immune formula. to save lives … at least until a vaccine is approved. But such a temporary cure was a setback last week when the U.S. Food and Drug Administration (FDA) suspended granting an emergency authorization for use as a remedy for COVID-19.
Not all chances of using plasma cure are closed, said Anand Shah, M.D., deputy commissioner for Medical and Scientific Affairs at the FDA. “The safe use of convalescence plasma is available to patients across multiple pathways, adding clinical trials, a national extended access protocol, or a new emergency application of an experimental drug for an unmarried patient.” In other words, it is much harder to find a remedy if you are in poor health and expect this specific remote possibility to save you.
For the latest news and recommendations on coronavirus, visit AARP.org/coronavirus.
The ruling came after researchers at the Mayo Clinic published early on the effects of a clinical trial of treatment in more than 35,000 adults, the maximum of them in critical condition, as a component of the U.S. Extended Plasma Access Program (EAP). Its effects showed that receiving previous plasma (as well as receiving plasma with more antibodies) was related to a decrease in the mortality rate.
Specifically, its researchers reported that the seven-day mortality rate in patients transfused with remedy within 3 days of diagnosis of COVID-19 was lower (8.7%) compared to patients transfused with plasma after 4 days of diagnosis (11.9 percent).
However, as recognized by the Mayo Clinic, the test has still been peer-reviewed or peer-reviewed to determine its validity. Mayo researchers say their purpose in publishing this initial knowledge “is to advance the discussion on the effectiveness of convalescent plasma treatment” and “recognize that additional clinical studies are needed.”
Save 25% by joining AARP and registering for auto-renewal for the first year. Instant access to the discounts, programs and data you want to enjoy all the spaces of your life.
News of the suspension of plasma use as an emergency remedy temporarily sparked debate and confusion this week.
Arthur Caplan, director of the medical ethics department at New York University’s Grossman School of Medicine, said the FDA has the right to eliminate the use of emergency therapy. “I think there has been concern that there has been too much panic prescription and lack of organization to check new things. When you use them as a component of an exemption or as a component of a compassionate use topic, you do not examine it. “That the FDA terminates emergency use in favor of a strict clinical trial protocol,” he said, is an effort to “regain public trust.”
The use of convalescence plasma is not new; the Spanish influenza pandemic was used to save lives. It has been studied for use in small trials in COVID-19 patients in China and the Netherlands.
In the United States, researchers across the country are not abandoning the remedy and continue to participate in a gigantic nationwide rigorous clinical trial to compare the protection and efficacy of convaletic plasma healing with placebo in adults exposed to COVID-19Array The double-blind examination is sponsored through the National Institutes of Health (NIH) through the U.S. Department of Defense.
Edward ‘Lalo’ Cachay, MD, professor of medicine in the Division of Infectious Diseases and Global Public Health at the University of California School of Medicine, San Diego, which is one of the verification sites, says: “I think what we know so far, the use of convalescence plasma is safe for other people with COVID-19 who get it in general. Can it be said how it should be and how it should be effective? Is it safe for other seriously ill people? We don’t have the data, we don’t know.
He says that until scientists have more powerful data, “we won’t be able to draw conclusions and recommendations.”
Please comment below.
You want to sign in to leave a comment.
Will WW extend a custom weight loss plan?
25% discount on the delivery of the first meal of $99 or more.
Giving or receiving assistance for the coronavirus pandemic
AARP members get exclusive benefits and social change.
AARP is a non-partisan non-profit organization that allows others how they live as they age.
You leave AARP.org and move to our trusted provider’s website. The terms, situations, and policies of the provider apply. Go back to AARP.org to learn more about the other benefits.
You leave AARP.org and move to our trusted provider’s website. The terms, situations, and policies of the provider apply. Go back to AARP.org to learn more about the other benefits.
You leave AARP.org and move to our trusted provider’s website. The terms, situations, and policies of the provider apply. Go back to AARP.org to learn more about the other benefits.
You leave AARP.org and move to our trusted provider’s website. The terms, situations, and policies of the provider apply. Go back to AARP.org to learn more about the other benefits.
You leave AARP.org and move to our trusted provider’s website. The terms, situations, and policies of the provider apply. Go back to AARP.org to learn more about the other benefits.
You leave AARP.org and move to our trusted provider’s website. The terms, situations, and policies of the provider apply. Go back to AARP.org to learn more about the other benefits.
You leave AARP.org and move to our trusted provider’s website. The terms, situations, and policies of the provider apply. Go back to AARP.org to learn more about the other benefits.
You leave AARP.org and move to our trusted provider’s website. The terms, situations, and policies of the provider apply. Go back to AARP.org to learn more about the other benefits.
You leave AARP.org and move to our trusted provider’s website. The terms, situations, and policies of the provider apply. Go back to AARP.org to learn more about the other benefits.
You leave AARP.org and move to our trusted provider’s website. The terms, situations, and policies of the provider apply. Go back to AARP.org to learn more about the other benefits.
You leave AARP.org and move to our trusted provider’s website. The terms, situations, and policies of the provider apply. Go back to AARP.org to learn more about the other benefits.
Your email is now confirmed.
Manage your personal email tastes and tell us what topics you are interested in so that we can prioritize the data you receive.
Find out what AARP has to offer.
Javascript must be enabled to use this site. Enable Javascript in your browser and check again.