A leading company oriented to virtual transformation.
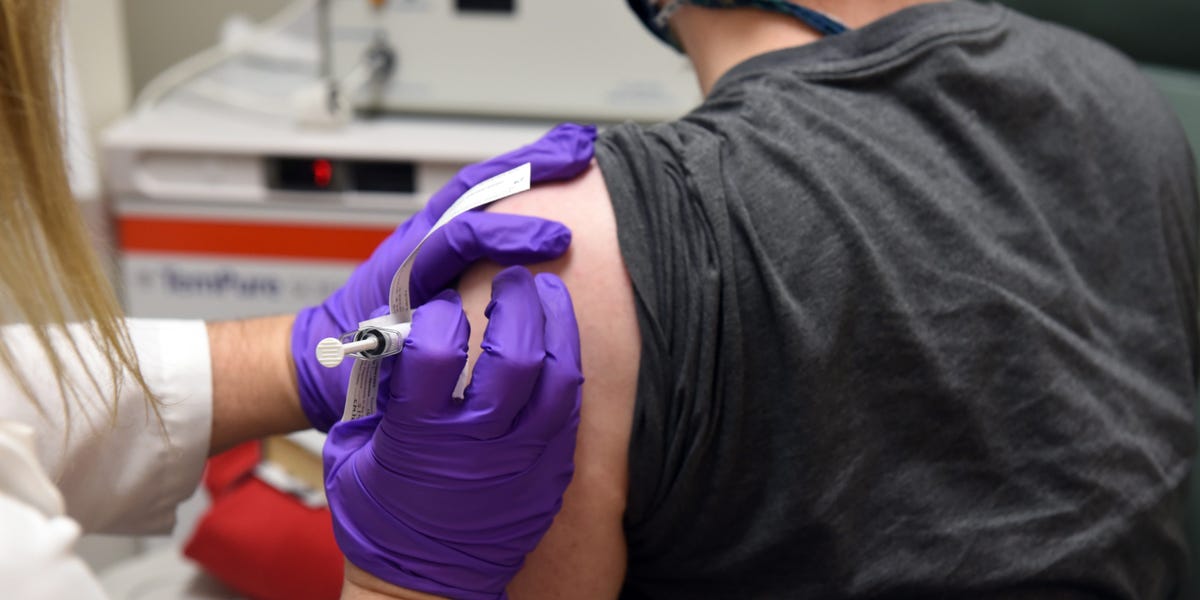
The European Medicines Regulator has taken steps that can boost the approval of a COVID-19 vaccine developed through BioNTech of Germany and the US pharmaceutical organisation Pfizer.
A new “continuous review” presented Tuesday through the European Medicines Agency will assess the effectiveness of the vaccine in real time as knowledge goes back to patient trials. This means that the company will wait for BioNTech and Pfizer to complete their trials and present all the knowledge at the same time.
The review can therefore speed up approval of the vaccine if it turns out to be effective in the end.
The firm said Tuesday that it has begun reviewing knowledge of laboratory studies on the vaccine and will continue to compare knowledge until it has enough to make a final decision, he said.
The resolution to initiate an ongoing review of BioNTech and Pfizer’s COVID-19 vaccine is based on the effects of past clinical trials in adults who found that the vaccine causes an immune response, the firm said.
The vaccine is the COVID-19 candidate at the time it is approved for ongoing review through the EMA. The firm showed on Thursday that an ongoing review of the vaccine developed through AstraZeneca and oxford University had begun. This follows a pause in vaccine trials. after a player reported severe neurological symptoms, the UK resumed testing, while the US resumed testing. The U. S. is still investigating.
The Pfizer vaccine is found in the later stages of examination in the United States, Brazil, South Africa and Argentina.
The BioNTech and Pfizer vaccine may be only one of the first COVID-19 applicants to be approved in Europe. The vaccine race on the continent is more urgent as the rate of infection increases.
The record for coronavirus deaths surpassed one million on September 29.
Get the latest research on the economic and advertising effect on Business Insider Intelligence coronaviruses on how COVID-19 affects industries.