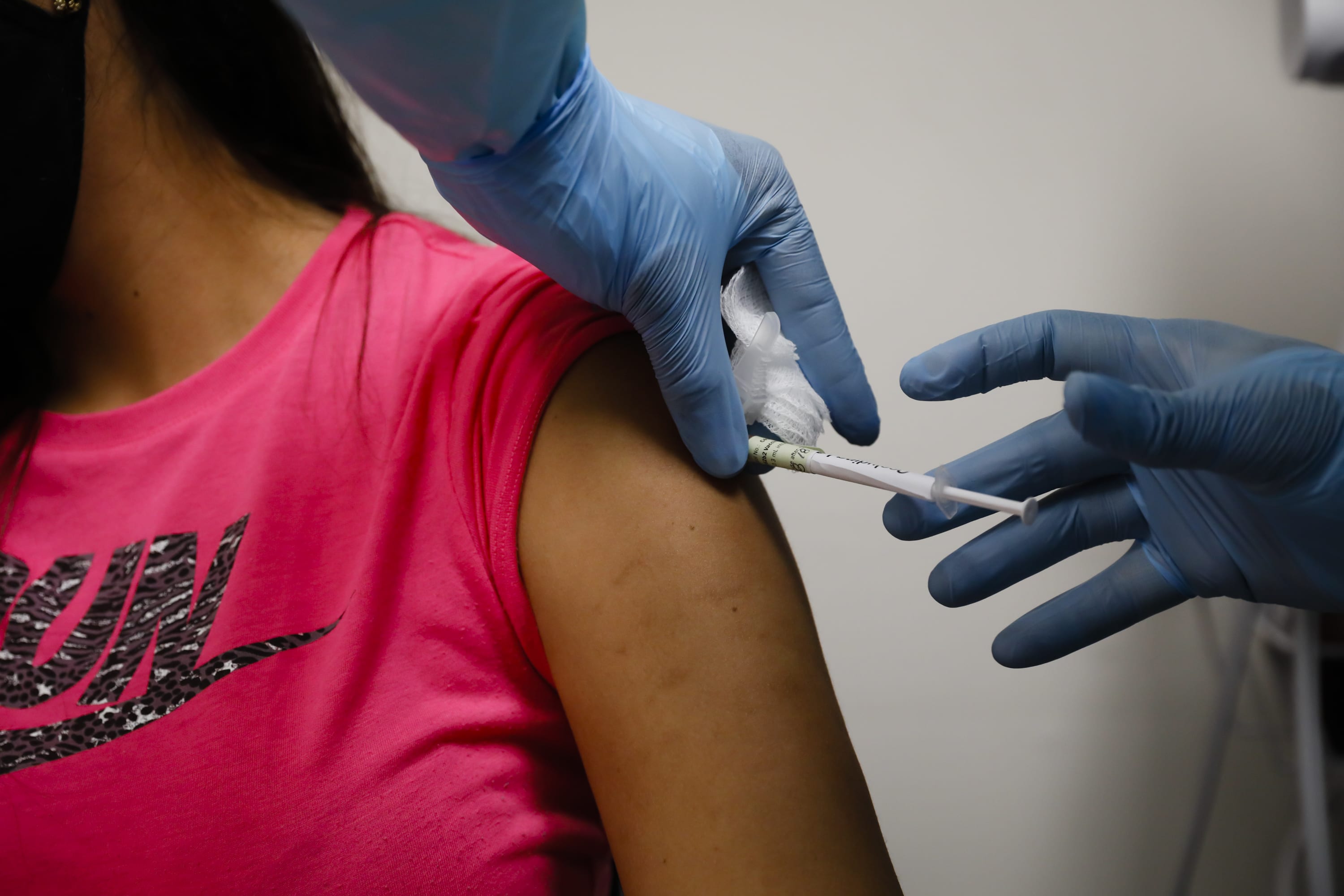
The European Health Regulator is examining in real time a COVID-19 vaccine developed through Pfizer and BioNTech, a few days after the launch of an evaluation procedure for the AstraZeneca vaccine.
The European Medicines Agency (EMA) said Tuesday that its committee on medicines for human use compares the first batch of vaccine knowledge and will continue to do so until sufficient knowledge is available to make a final decision.
Pfizer and BioNTech said in a set that the start of the review was based on knowledge of laboratory and animal testing, as well as early human testing, while discussions were being held to present knowledge as they emerged.
The EMA uses “continuous reviews” to expedite pandemic vaccine testing by reading knowledge as it is submitted, rather than waiting for all knowledge to be gained with a formal application.
Last week, he began examining the vaccine of AstraZeneca and the University of Oxford, AZD1222 or ChAdOx1 nCoV-19, expanding the chances that the British vaccine will be the first to be approved in the region for the disease that has killed more than one million people worldwide.
While AZD1222 uses a weakened edition of a chimpanzee bloodless virus to bring to life the immunity opposed to Covid-19, the Pfizer and BioNTech vaccine, called BNT162b2, uses ribonucleic acid, a chemical messenger ordering protein production.
When injected into humans, BNT162b2 asks cells to produce proteins that mimic the outer surface of the new coronavirus, which is framed as a foreign invader and generates an immune reaction opposite to it.
The Pfizer vaccine is currently being evaluated in complex studies in the United States, Brazil, South Africa and Argentina.
Do you have any confidential information? We want to hear from you.
Sign up for loose newsletters and get more CNBC in your inbox
Get it in your inbox and more information about our services.
© 2020 CNBC LLC. All rights are reserved. An NBCUniversal department
Knowledge is a real-time snapshot: data is delayed for at least 15 minutes, monetary and global industry news, inventory quotes, and market knowledge and analysis.
Data also by