Quinn Eastmanqeastma@emory. edu
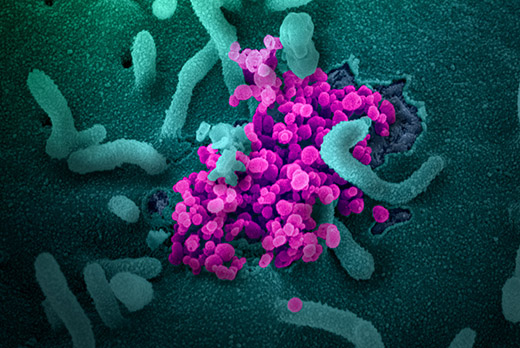
SARS-CoV-2 scanning electron microscope symbol emerging from the surface of laboratory cultured cells.
Credits: NIAID-RML
All the stories of emory COVID-19
Emory is helping to identify national care for COVID-19 October 15, 2020
Emory experts should talk about COVID-19
Health, Research, School of Medicine, Woodruff Center for Health Sciences, Clinical Trials, Coronavirus, Research in Health Sciences, Immunology, Infectious Diseases, Vaccines
Emory University will recruit adult volunteers in a large-scale clinical trial designed to assess whether a single dose of an experimental vaccine can prevent symptomatic COVID-19.
Vaccine candidate, evolved through Johnson’s Pharmaceutical Janssen
This is the first complex-level trial to assess whether a single-dose vaccine can be opposed to COVID-19 to others.
“We are excited to have the opportunity to conduct this phase 3 clinical trial of the Janssen adenovirus vector COVID-19 vaccine,” said Dr. Evan Anderson, principal investigator of the Emory trial and professor of medicine and pediatrics at Emory University School of Medicine and Child Health Care in Atlanta.
“COVID-19 has caused hospitalizations and deaths in the state of Georgia. This new vaccine has wonderful potential to fight the pandemic if it is safe and effective. “
Vaccine candidate Janssen uses weakened human adenovirus to make the complex SARS-CoV-2 protein explicit in cells. Adenoviruses are viruses that cause colds. However, the vector of adenovirus used in janssen’s vaccine candidate has been changed to be reflected in humans and causes disease.
The main goal of the Phase 3 trial is to determine whether the experimental vaccine can save you moderate to severe COVID-19 after a single dose. The trial will use specialized tests to distinguish between immunity from herbal infection and vaccine-induced immunity.
Janssen, the National Institute of Allergy and Infectious Diseases (NIAID) and the Advanced Biomedical Research and Development Authority (BARDA) are investing the trial.
The Janssen vaccine is the fourth candidate of its kind in the last stages of testing in the United States. The Janssen vaccine trial will recruit up to 60,000 volunteers at more than two hundred clinical studies sites in the United States and around the world. Some of the sites are components of the COVID-19 Prevention Network (CoVPN) supported through NIAID, a collaboration of 4 existing clinical study networks, adding the Emory-based Clinical Infectious Diseases Research Consortium.
Volunteers must give their informed consent to participate in the trial. After offering a nasopharynx and blood reference pattern, participants will be randomly assigned to obtain a dose of the prospective vaccine or a saline placebo. The trial is blind, which means that neither researchers nor participants will know who is receiving the experimental vaccine.
Participants will be strongly monitored for protection reasons and asked to provide more blood samples at express times after injection and for two years. Scientists will analyze blood samples to find and quantify immune responses to COVID-19.
Adults who wish to enroll in this exam may scale into the COVID-19 prevention network.
Emory is also completing a phase 3 trial for an experimental vaccine opposed to the new coronavirus.
In addition to vaccine trials, Emory researchers are conducting a multitude of studies to diagnose, treat and save the new coronavirus, adding research on monoclonal antibody therapies.
COVID-19 Prevention Network Paintings (CoVPN) created through the National Institute of Allergy and Infectious Diseases (NIAID) of the US National Institutes of Health. But it’s not the first time To respond to the global pandemic. Through CoVPN, NIAID leverages the infectious disease expertise of its existing netpaintingss studies and global partners to address the urgent need for vaccines and antibodies that oppose SARS-CoV-2. CoVPN is headquartered at the Fred Hutchinson Cancer Research Center. For more information, visit the COVID-19 Prevention Netpaintings website.
IdRC, composed of the Vaccine and Treatment Assessment Units (VTEU) and the IDRC Leadership Group, was formed in 2019 to develop plans and implementation of clinical studies on infectious diseases that meet NIAID’s clinical priorities. The consortium includes leaders in infectious diseases and clinical studies. Emory University, University of Maryland School of Medicine, Baylor College of Medicine, Cincinnati Children’s Medical Center and University of Cincinnati, FHI360, Fred Hutchinson Cancer Research Center, Johns Hopkins University, Kaiser Permanente Washington Health Research Institute, New York University, St. Louis University Array Vanderbilt University Medical Center, University of Alabama at Birmingham, University of Rochester, University of Washington and NIAID.