The online symposium “The Role of Cytokines in COVID-19” was presented on June 18, 2020 through the NIH/FDA Immunology and Cytokines interest teams and aimed to talk about our rapidly evolving understanding of COVID-19-like cytokine responses at other stages of infection, adding etiologies, subsequent consequences and imaginable mitigation strategies. Recording can be obtained in https://nci.rev.vbrick.com/sharevideo/03106730-66cc-47ba-870b-f6e6274a998a.
The symposium was opened by Anthony Fauci, Director of the National Institute of Allergy and Infectious Diseases at the U.S. National Institutes of Health (NIAID, NIH), and Janet Woodcock, Director of the Center for Drug Evaluation and Research, Food and Drug Administration (FDA) and currently heads the healing component of Operation Warp Speed. Fauci briefly reviewed the current status of the 2019 coronavirus pandemic (COVID-19), noting that the global occurrence had more than 8 million cases and more than 300,000 deaths, with more than 120,000 deaths in the United States alone (occurrence as of June 18, 2020). The causal virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a single-stranded RNA virus that uses the angiotensin 2 conversion enzyme (ACE2) as a cell receptor. The atomic conformation of the virus’s complex prefusion protein was recently described by scientists and colleagues at the NIAID1 Vaccine Research Center. He also highlighted the role of cytokines in the pathogenesis of various clinical presentations of COVID-19, ranging from asymptomatic to pneumonia, neurological disorders, acute respiratory misery syndrome (SED), cardiomyopathy, sepsis, hypercoagulability, multiorgan failure and death, as well as multisistemic inflammatory syndrome observed in children. In addition, the advantages of the dexamethasone remedy in severe coVID-19 cases requiring ventilation were discussed, which is consistent with the central roles of inflammation and a cytokine typhoon in the cause of a serious pathology. Fauci concluded his speech by drawing attention to the many projects undertaken and supported through NIAID to combat the COVID-19 epidemic. Woodcock followed with a speech that highlighted the wide variety of COVID-19 clinical presentations, underlining the central role of the immune reaction in this disease. He also pointed to the obvious geographical teams of disease manifestations and the desire to better perceive the imaginable points of host-pathogen interactions beyond known conditions of fitness of the past, such as past innate immune delight and sophisticated differences in ACE2 expression in populations. Finally, he discussed the complexity of knowledge of multiple clinical trials that focus on the inflammatory procedure underlying the disease, emphasizing the importance of building clinically applicable biomarkers to advise the healing course.
The first clinical consultation of the assembly focused on the evolution of the reaction of COVID-19 cytokines and was opened through Miriam Merad (Mount Sinai School of Medicine). Merad first reminded us that the infection is asymptomatic in 80% of adults and at most in children, however, 20% of patients require hospitalization in the intensive care unit (ICU). The mortality rate of ICU patients is 25%, with maximum deaths attributed to severe inflammation and embolic complications. According to Woodcock’s comments on the importance of biomarkers in increased targeted healing efforts, Merad described studies using higher-dimensional profiles to identify early markers awaiting the severity of the disease. In one study, his organization decided on a platform that monitors interleukin (IL) -6, IL-8, IL-1 and tumor necrosis (TNF), which are well-established targets for anti-inflammatory treatments. They tested more than 1,500 patients on the day of hospitalization and then correlated serum cytokine concentrations with the outcome of the disease. His knowledge showed that IL-6, IL-8 and TNF and, to a lesser extent, IL-1, were the first at the time of hospitalization and their concentrations correlated with disease and mortality, even after correction based on age, ethnicity, race and comorities, suggesting that they can only be used to identify patients with threat of serious illness2.
Pro-inflammatory cytokines remained elevated throughout the course of the disease unless patients were treated with steroids or repopulated, reducing circulating IL-6 levels. It also noted that IL-6 and TNF are regulated independently and can therefore be targeted in parallel in patients with serious illnesses. Merad then described the first effects of deep-profile longitudinal studies using general blood proteomics known to 23 groups of cytokines that are expressed differentially in patients with benign disease, severe disease without terminal organ damage, and serious disease with terminal organ damage. In this broader evaluation, patients with a severe disease had a buildup of IL-6 and other pro-inflammatory cytokines, while those with a moderate disease had a tendency to suggest T-cell priming. Finally, Merad cautioned that the reaction style of cytokines in young people with multisystem inflammatory syndrome had a similar component to that of adults with a serious illness because they had a growing expression of pro-inflammatory cytokines; however, they also had a variety of autoantibodies, adding some traditional autoantibodies, but also autoantibodies directed in opposition to cardiac and endothelial antigens that may be only the manifestations of the disease. It is vital to note that the cytokine profiles provided at the time of hospitalization in young people and adults have been maintained throughout the course of the disease unless modified by a remedy with steroids, mediaivir or immunomodulatory cure and may simply be correlated with the disease. end results of the disease suggesting they can be used. to advise the healing approach.
John Tsang (NIAID, NIH) then discussed the application of COVID-19 systemic immunology research. He described studies using mobile indexing of transcriptomas and epitopes by sequencing (CITE-seq), a mobile single-mobile research approach that combines detection of multiplexed surface protein markers at the top with the transcriptome profile. Tsang’s laboratory and his colleagues evaluated the mononucleated peripheral blood mobiles (PBMC) of a longitudinal cohort of patients hospitalized with COVID-19 in Brescia, Italy, taken at the height of the epidemic in this region. Patients evaluated in this first experiment included thirteen severe or critical patients and five healthy controls paired by age and gender. Two specific s emerged that differed from the conclusions reported by other researchers. First, while previous studies have recommended that the virus disrupt the type I interferon signaling pathway (IFN), fueling the hypothesis that the absence of an early reaction of type I (and III) IFN allows immediate viral spread and induces hyper-inflammatory reactions, this study a signature of transparent type I IFN in key immune moving subsets in patients with COVID-19. These effects recommend that a careful assessment of patient populations tested through other studies be carried out, as delays in inducing such reactions may differ depending on the level of the disease and may be more influenced by genetic and environmental factors. In addition, to date, Tsang has not discovered any evidence of T cell phone depletion, as expanded CD8-T mobile clones have been found to have reduced EXPRESSION of PD-1 compared to undeveloped mobiles in patients. This differs from other studies that discovered maximum amounts of PD-1 expression in lymphocytes3, indicating exhaustion and raising the option that blocking checkpoints may be considered as treatment.
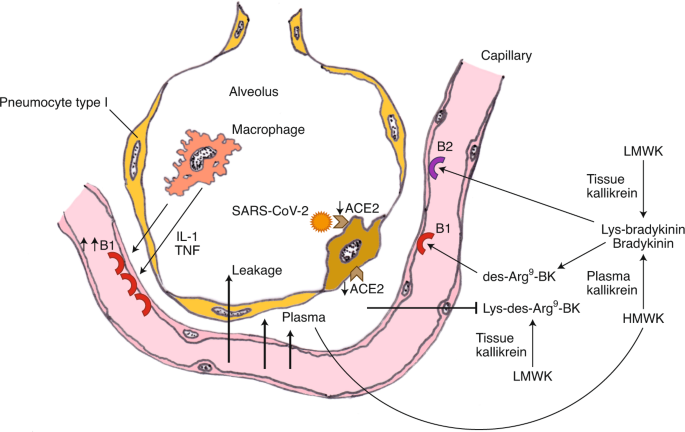
The final seminar in this session was given by Frank van de Veerdonk (Radboud University Medical Center). The main focus of his seminar regarded unique aspects of COVID-19 immune pathology; specifically, the role of ACE2 in modulation of the kallikrein–kinin system. Preliminary results suggested that this system has a role in the angioedema observed in the lungs of COVID-19 patients. Biological pathways were evaluated via proteomic analysis of serum from patients with severe (necessitating ICU care) versus more moderate (not requiring ICU care) disease, with an initial focus on IL-1 biology and autoinflammation and a subsequent focus on the kallikrein–kinin system. IL-6 upregulation was prominent in patients with severe disease, which van de Veerdonk proposed was a manifestation of the strong induction of an autoinflammatory loop via IL-1β and the IL-1 receptor (IL-1R). He proposed that, following SARS CoV-2 binding to pathogen recognition receptors, pro-IL-1β is induced, processed to IL-1β by activated inflammasomes and stimulates IL-6 and IL-18 production (from pro-IL-18). IL-1β binds to IL-1R on monocytes/macrophages, generating the autoinflammatory loop and recruitment of polymorphonuclear leukocytes. He also noted that IL-1α released during tissue damage may further contribute to the autoinflammatory loop by binding and signaling through IL-1R, inducing more IL-1β. He further remarked that IL-1 is a difficult cytokine to measure (echoing others) and that concentrations in plasma likely do not reflect those in the lung, suggesting that bronchoalveolar lavage (BAL) measurements would be important in verifying its upregulation at the site of primary pathology. Lastly, he remarked on the substantial reductions in IL-7R and stem cell factor and their potential relevance to the commonly observed lymphopenia in patients with severe disease, which is of clear interest from a therapeutic standpoint. In the last part of his talk, van de Veerdonk focused on the reduced amounts of serpin family A member 12 (SERPINA12) and DPP4 (CD26) in patients in the ICU versus those not in the ICU. Since these factors suppress inflammation mediated by kallikreins and it is expected that there is positive, coordinated stimulation of the kallikrein–kinin system from increases in IL-1 and IL-6 as well as from activated complement, the upregulation of the kallikrein–kinin system was investigated. Most critically, kininogens processed by kallikrein, a serine protease, will generate bradykinin (BK) or Lys-BK (Fig. 1), which can be further metabolized by tissue carboxypeptidases, producing des-Arg9-BK (DABK). DABK binds to bradykinin 1 receptors (B1Rs) and enhances vascular permeability, which can lead to local angioedema. Critically, DABK is inactivated by ACE2. Because ACE2 is internalized by SARS-CoV-2, it is no longer present to counter the downstream effects of DABK, thus producing angioedema in the lung, which potentially explains the early ARDS manifestation in these patients. Furthermore, IL-1 and inflammation in general stimulate increased expression of B1Rs, thus upregulating these receptors in the inflamed lung and boosting tissue-level angioedema. The ensuing plasma leakage into the alveolar space allows activation of plasma kallikrein–kinin at the site of infection. Subsequent production of bradykinin results in persistent angioedema via bradykinin activation of the bradykinin 2 receptor (B2R).
The negative regulation of ACE2 through SARS-CoV-2 is followed by the loss of the ability to neutralize Lys- [de-Arg9] -bradykinin (BK) in the lung, resulting in plasma loss. Subsequently, plasma leakage occurs in more B1R ligands (de-Arg9-BK) and B2R ligands (bradykinin), vascular permeskill and angioedema. CPM, carboxipepidatea M; CPN, carboxipeptidasa N; HMWK, a superior molecular weight cycinogen; LMWK, low molecular weight cycinogen. Figure reproduced with permission of the ref. 4, Publications by eLife Sciences.
This led to speculation that the calicrine-cinine formula was critically involved in the lung pathology of COVID-19, which was tested in the treatment of patients with hypoxia and increased oxygen needs with icatibant, a B2R blocker approved for hereditary angioedema (AOH). Promising effects have been observed and will be published shortly. This discovery suggests the need to expand testing, as well as the search for a more durable agent, lanadelumab, which blocks the plasma activity of calicrein and is approved for HAE. Based on the effects of this review and knowledge from other sources, a remedy protocol based on the level of the disease was proposed4.
At the time he began his consultation with Chen Dong (Tsinghua University), who spoke about his recently published work5 on humoral reactions in COVID-19 patients. In a small cohort, your organization seamlessly detected IgG1 and IgM antibodies that identified the recombinant nucleoprotein of SARS-CoV-2 and the complex protein receptor binding domain (S-RBD) in patients’ serum as opposed to the serum of healthy Americans. Overall, antigen-specific antibody concentrations were higher in Americans who had recently been discharged from the hospital (8 patients) compared to those controlled 2 weeks after discharge (6 patients). Longitudinal cohort studies of larger patients will be needed to find out the evolution over time and duration of fun reactions during COVID-19. Luigi Notarangelo (NIAID, NIH) noted in an examination of more than three hundred patients from Brescia and Monza in northern Italy that the proportion of plasmablasts in the blood was higher in the early stages of COVID-19 and decreased with the disease. Class 6. Using a neutralizing control discovered in pseudovirus particles, Dong found that thirteen of the 14 patients developed varying degrees of neutralizing antibodies with S-RBD specificity. In an independent study, 94% of the 175 patients developed neutralizing antibodies, suggesting that these funny reactions are a constant feature of COVID-197. More importantly, antibody titers did not correlate with the duration of the disease in this study, leaving open the question of the contribution of antibodies to determine disease progression. It is conceivable that an antibody threshold point is in synergy with other characteristics of immune reactions (e.g., Mobile activation T and reminiscences formation) and inflammatory to have effects on disease resolution. It is also not known to what extent neutralizing antibodies persist in convalescent Americans and whether they will be protected during reexposure to SARS-CoV-2. In this regard, another study reported that 40% of asymptomatic Americans and 13% of symptomatic Americans have become HIV-negative 8 weeks after hospital discharge6. This supposedly short-lived antibody reaction contrasts sharply with SARS-CoV-specific detectable IgG 2 years after infection8.
Notarangelo provided a comprehensive review of mobile and cytokine adjustments in the context of disease evolution and severity in the Italian cohort. A recurring trend in COVID-19 is the increased expression of pro-inflammatory cytokines such as IL-6, TNF and IL-1, as well as an IFN signature highlighted through the expression of downstream cytokines CXCL9; Similar results were also obtained through Merad and Tsang in session 1. Serum concentrations of endothelial mobile activation soluble biomarkers (ICAM-1 and VCAM-1) and septic surprise (lipopolysaccharides binding protein (LBP) and sIL-33R) also increased particularly in patients with COVID-19 and even more in those with critical disease who eventually died. In an attractive twist, Notarangelo showed that, during the course of the disease, some inflammatory markers, such as IL-6, did not replace particularly, while others, such as IL-33R soluble (sIL-33R) and CXCL10, minimized in patients who eventually recovered but remained steadily increased in those who succumbed to COVID-19. In addition, cytokines related to myeloid differentiation were definitively correlated with the severity of the disease, suggesting a contribution of de novo myelopogenesis. A striking aspect in this study was the small number of CD4 and CD8 T mobiles in patients with COVID-19. T lymphopenia was noticeable even in patients with mild symptoms and reached excessive deficits in those with severe illness9. The underlying cause of T-mobile loss is not known and will be a vital domain of long-term research. Notarangelo also highlighted the unexpected minimization of soluble Fas ligand (sFasL) and sCD62L, in contrast to the maximum concentrations of sCD25, a T-mobile activation biomarker. A prepress in bioRxiv also reported deep activation of antigen-specific T-mobiles and cytotoxic responses even in HIV-negative individuals7. More studies are needed to perceive the contributions of such immune responses contrasting to the evolution of COVID-19. Finally, Notarangelo provided evidence that relief in the expression of HLA-DR and CD4 in peripheral blood monocytes (the former a biomarker of the ability to present antigens) correlated with the severity of the disease9. By changing the antigen presentation and the upcoming activation of T mobiles, this mobile phenotype can mitigate effective responses during SARS-CoV-2 infection. The predictive prospect of this biomarker remains to be determined.
The last speaker of this session, Xiaoyu Hu (University of Tsinghua), evolved in particular on the nature and origins of cytokine responses in COVID-19, focusing on the pulmonary myeloid compartment of patients. Previous studies have shown that the largest population of alveolar macrophages living in the lungs is of fetal origin and is maintained through proliferation in situ10. Single-mobile RNA sequencing studies of BAL fluid in PATIENTS with COVID-19 showed a very large flow of monocyte-derived peripheral macrophages to the lungs, with proportional relief in the frequency of alveolar macrophages; these adjustments in moving populations correlated with the severity of the disease11,12. Recruited macrophages basically expressed maximum amounts of chemokcins such as CCL2, CCL7 and CCL8 and, with the severity of the disease expanding, CXCL10 and CCL3. In contrast, typical pro-inflammatory cytokines, such as IL-6 and IL-8, which were increased to the outer edge, were not regulated higher upwards on those mobiles. Separate lung-specific responses would possibly contribute to COVID-19 pulmonary pathophysiology, for example through CCL211-mediated macrophage recruitment.
The basis of the dichotomy between the reaction of pulmonary macrophages governed by chemokcins and the peripheral prevalence of more traditional inflammatory cytokines is unclear. One option is that the pulmonary microenvironment distorts macrophage reactions to chemokine gene expression. In this scenario, the reactions of pulmonary macrophages in other acute respiratory syndromes are also asymmetrical. However, it should be noted that resident alveolar macrophages do not provide this model, indicating that this is not just a microenvironment problem. Alternatively, SARS-CoV-2 infection can generate a unique inflammatory medium that promotes the observed gene expression pattern. The fact that it is limited to recruited macrophages indicates a role for peripheral priming in the generation of phenotypes.
The symposium concluded with a consultation discussing recent progress in the management of viral infections and cytokine typhoons related to biologics and medicines to treat the disease and how these approaches have made particular progress in the outcome of the disease. Randy Cron (University of Alabama at Birmingham) began this consultation by recalling that, long before COVID-19 entered the spotlight, the cytokine typhoon was already identified as a major challenge in the picture of immune homeostasis. Cytokine typhoon is a generic term for several hyper-inflammatory immune responses that accompany cytokine release syndrome, negative culture septicaemia, macrophage activation syndrome and hemophylotic lymphohicytosis (HLH), among others. Therefore, there are several causes of the cytokine typhoon, which can be triggered by genetic factors, cancer, viral infections and other aggressions and that want to be controlled accordingly. Therefore, the cytokine typhoon triggered by COVID-19 deserves to be manageable wisdom accumulated in the beyond founded on the control of similar viral infections (Figure 2). During his brief review of the literature, Cron cited an examination beyond that the inability of perforin-deficient CDTTS to remove LCMV-infected target cells resulted in the death of mice infected with overactivation, but not when IFN13 was neutralized.
A graphical representation of disease evolution by up to 20% of others with COVID-19 that expand cytokine typhoon syndrome (CSS) and respiratory misery that require hospitalization17. The severity of the disease occurs along the y-axis and the time in days along the x-axis. The participation of innate and adaptive immune responses is in the form of orange and green triangles, respectively, the other stages of the disease. The early stages of infection (full-size image
In this sense, the alteration of other genes involved in the cytolytic pathway also occurs in a cytokine typhoon and fatal hyperinflation. For example, the Cron organization sewn the entire exomame of patients who succumbed to H1N1 influenza infection and found that 36% of the other 14 people in the study had mutations in the genes that encode perforin or the lysosomal traffic regulator (LYST). Therefore, the cytokine typhoon is clearly related to the failure of the removal of target cells inflamed with the virus and persistent stimulation of T cells. As a solution, Cron summarized some approaches to IL-6, IL-1 or JAK/STAT proteins to suppress the cytokine typhoon. He concluded his presentation by proposing methods of choice for decreasing hyperinflamation, such as JAK inhibitors and glucocorticoids. Specifically, the World Health Organization first recommended not to use COVID-19 corticosteroids discovered in SARS and MERS experiments, however, recent knowledge has shown marked relief in the need for ventilation and mortality in COVID-19 patients treated with dexamethasone14. available and affordable, and with a careful timing and timing, these agents can simply be a resilient equipment to combat the cytokine typhoon caused by SARS-CoV-2.
At the next seminar, Eleanor Fish (University of Toronto) brought the COVID-19 pandemic into attitude, highlighting two other coronavirus outbreaks that preceded SARS-CoV-2, SARS-CoV in 2002 and MERS-CoV in 2012. For viral infections, the first 24 to 72 hours are the highest criticism, and Fish noted that the reaction of type I IFN through innate cells plays a central role in this process. Type I IFNs are resistant because they not only act directly on inflamed cells to suppress viral replication, but also recruit and activate immune cells to eliminate the virus. In particular, SARS-CoV-2 GENOMA encodes genes that inhibit IFN production, such as Nsp1, Nsp3, ORF6 and M proteins. Therefore, it is transparent that viruses are supplied with equipment to mitigate host IFN production, and SARS-CoV-2 and SARS-CoV do not cause an intelligent IN TYPE I reaction.
In this regard, the Fish organization demonstrated that replication of SARS-CoV in vitro can also be suppressed either by an alphacon-1 IFN remedy (an artificial IFN) and that this effect was replicated in a clinical trial when SARS patients were injected subcutaneously. with IFN alfacon-1, resulting in immediate pulmonary clearance and really extensive innovations across various clinical parameters. Translating these effects into COVID-19, a clinical trial was designed involving 77 hospitalized patients with shown cases of COVID-19 in Wuhan, China. In this trial, patients were treated with the antiviral drug arbidol (ARB), nebulized IFN-2b or a mixture of the two agents. Although researchers did not discover differences between patients in treatment equipment in terms of frame temperature, oxygen saturation or blood biochemistry, there was a significant difference in viral clearance, as patients treated with IFN eliminated the virus much more quickly. In particular, patients treated with IFN also showed particularly reduced concentrations of serum IL-6 and C-reactive protein (PCR) (Fig. 3) 15. Therefore, with caution that this trial was a study of small, non-randomized cohorts. , knowledge advised the use of TYPE I IFN as an early intervention agent in COVID-19. In addition, the IN TREATMENT type would possibly be more favorable than other remedies because it has not triggered a typhoon of cytokines or serious adverse events. In addition, it would possibly be advantageous compared to the remedy with type III IFN, given the formulaic spread of SARS-CoV-2 from the lungs to the vascular formula and other organs, since type I IFN receptors are expressed in virtually all cells, while expression of the type III IFN receptor is largely limited to epithelial cells.
Patients with COVID-19 shown were treated with ARB alone (ARB; 24 patients) or IFN-2b with or without ARB (IFN; 53 patients). On the left, the upper respiratory samples were evaluated by PCR for the presence of SARS-CoV-2. The proportion of patients with a detectable virus was shown to be based on the day of sampling from the onset of symptoms. The P-price of the remedy’s effect was assessed using a proportional Cox threat style that included age and comorities as covariates. On the right, samples were taken from patients in series to evaluate IL-6 from the day of onset of symptoms. Recorded prices were added in 3-day periods and presented as an average. The p-price was assessed using R v.3.6.0, and a variance research (ANOVA) was used to verify the effect of the remedy, adjusting age and comorities. Figure reproduced with permission of the ref. 15, International Union of Immunology Societies.
Michail Lionakis (NIAID, NIH) presented his recent paintings on a kinase inhibitor to counteract the COVID-1916 cytokine typhoon. The hyper-inflammatory reaction of COVID-19 includes activation of NF-B and inflammation (Nlrp3), as evidenced through accumulation in degrees of cytokines and pro-inflammatory chemokcins, adding IL-1, TNF, IL-6, CCL2 and CCL3. , among others. It therefore raised the hypothesis that neutralizing these cytokines or their downstream signalling would be an effective strategy for suppressing COVID-19-related cytokine typhoon.
Using mouse models with Bruton tyrosine kinase deficiency (BTK) and BTK pharmacological inhibitors, the Lionakis organization has in the past known the target population for BTK inhibition in host antifungal defense as macrophages and non-mobile B. ibrutinib is an effective and well-tolerated BTK inhibitor that blocks mobile receptor B signaling and is used as a remedy for malignant mobile tumors B and inflammatory situations such as graft-versus-host disease. Therefore, macrophage suppression was thought to be a side effect of BTK inhibition, resulting in impaired fungal immunity, as demonstrated by susceptibility to fungal infections in a subset of patients treated with ibrutinib. It has been significantly reported that ibrutinib suppresses macrophages by inhibiting the activation of inflammation and NF-B, exactly any of the downstream pathways of the cytokine typhoon in COVID-19 gravis. These findings led Lionakis and his colleagues to hypothesize that BTK inhibitors would improve hyperinflamation and therefore prevent clinical deterioration in patients with severe COVID-19. Lionakis’ organization hypothesized that the manifest immune reaction of SARS-CoV-2-infected alveolar macrophages would possibly be inhibited in proximal signaling by non-conforming use of a second-generation BTK inhibitor, acalabrutinib. This technique may also be simply amazing for the use of individual pro-inflammatory cytokine express neutralizing antibodies, as it can also potentially suppress the effect of several cytokines. In addition, because BTK is not expressed on T mobiles, the inhibition of BTK would restrict the effect to macrophages without affecting the effector that serves as antiviral T mobiles.
The effects of the first clinical examination with 19 patients were in line with the hypothesis, and some noticeable effects were observed in patients with COVID-19 under the pre-UCI16 framework. Although patients with severe symptoms of COVID-19 had increased production of ilocytes from IL-6 to CD14 in the blood, the remedy with acalabrutinib particularly advanced oxygenation, decreased inflammation (e.g. Reduced production of PCR and IL-6) and also particularly increased the number of lymphocytes in most patients. Mechanically, it was discovered that monocytes, but not B cells in patients with COVID-19, contained higher basal degrees of BTK phosphoryl, which validates that BTK inhibition targeted through monocytes/macrophages provides the basis for suppression of cytokines typhoons14. Currently, 3 other BTK inhibitors, in addition to acalabrutinib, zanubrutinib and ibrutinib, are recently undergoing clinical trials for the remedy of COVID-19 gravitation. Further studies will be needed to validate these clinical findings that, if confirmed, would potentially involve a wider use of BTK inhibitors in the control of hyper-inflammatory responses.
Overall, discussions in this consultation highlighted multifaceted approaches to neutralizing the cytokine typhoon in COVID-19 patients and presented the first successes. In addition, the extra effects illustrate the need for agile approaches in the implementation of intervention strategies, depending on the severity or level of the disease. Therefore, the dose, timing and variety of the patient organization deserve to be considered conscientiously and in the context of disease progression. Treatment with dexamethasone, for example, deserves to be reserved for complex patients due to their immunosuppressive function, while type I IFNs are obviously suitable for research in immediate and early treatment. Targeted suppression of immune mobile activities through BTK inhibitors has been tested in patients requiring oxygen supplements, but it is unclear whether the early level remedy would be even more effective.
This convention brought together more than 1,600 scientists from other countries and time zones, demonstrating the strength to organize a virtual convention, but also documenting the immense global interest in COVID-19 biology. Due to its wonderful good luck and enthusiastic feedback that has been received, the upcoming COVID-19 convention organized through the NIH/FDA Immunology Interest Group and the Cytokines Interest Group is already scheduled, and we expect broad participation in cyberspace in November. 2020 to share the newest and most exciting coVID-19 studios with the foreign community.
We thank the speakers for their valuable comments in the preparation of this assembly report. The perspectives expressed in this article are those of the authors and do not necessarily reflect the policy or official position of the U.S. Food and Drug Administration. And the Department of Health and Human Services, or advertising names, advertising products or organizations. require approval from the U.S. government.
Cell Immunology Section, Immune System Biology Laboratory, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Maja Buszko
Experimental Immunology Branch, Cancer Research Center, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Jung-Hyun Park
Innate Immunity Laboratory, Biotechnology-III Review and Research Division, Office of Biotechnological Products, Drug Evaluation and Research Center, U.S. Food and Drug Administration, Silver Spring, MD, USA.
Daniela Verthelyi
Laboratory of Molecular Biology and Immunology, National Institute of Aging, Baltimore, MD, USA
Ranjan Sen
Cancer Immunometabolism Laboratory, Cancer Research Center, National Cancer Institute-Frederick, Frederick, MD, USA.
Howard A. Young
Immunology Laboratory, Biotechnology-III Review and Research Division, Office of Biotechnology Products, Drug Evaluation and Research Center, U.S. Food and Drug Administration, Silver Spring, MD, USA.
Amy S. Rosenberg
The authors claim to have competing interests.
Published: 27 August 2020
DOI: https://doi.org/10.1038/s41590-020-0779-1