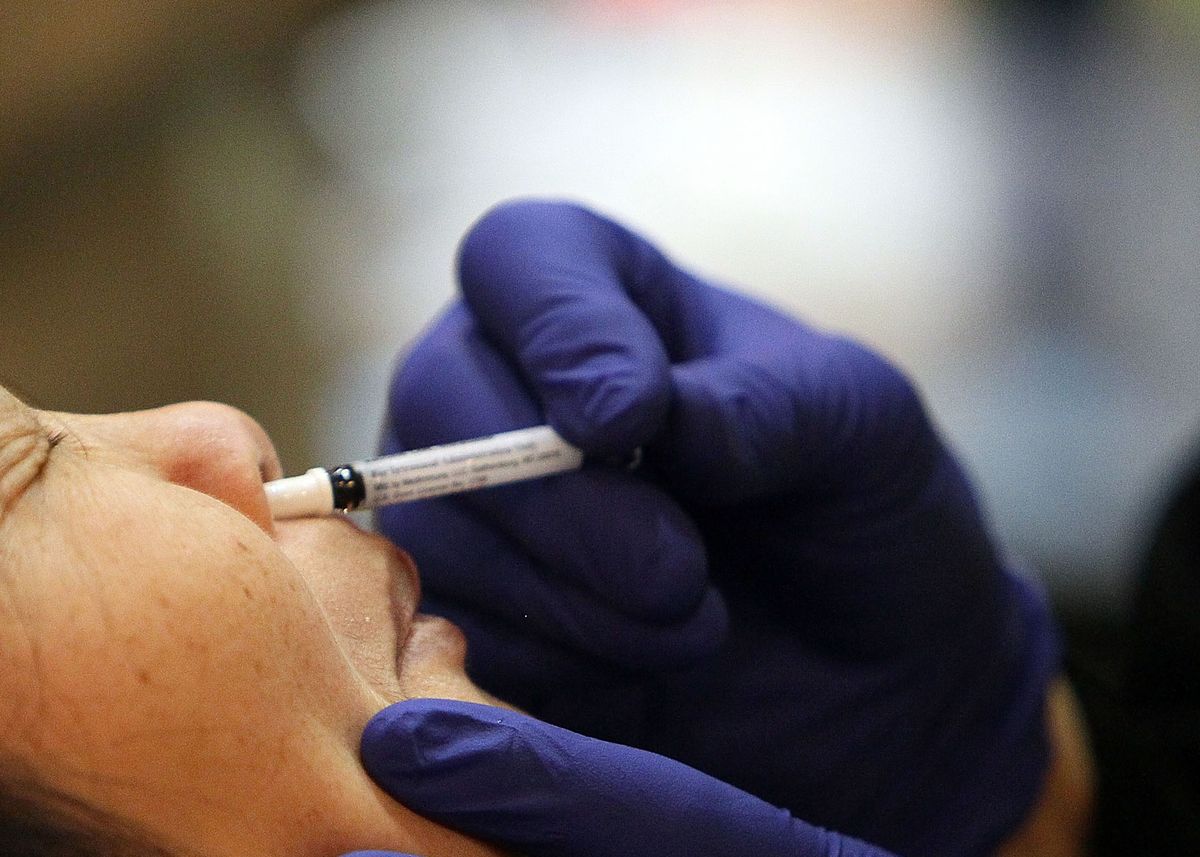
A dual experimental vaccine that opposes influenza and the new nasal spray coronavirus will enter human studies in Hong Kong next month, a leading doctor in infectious diseases said.
The early-stage clinical trial will recruit about a hundred adults, said Yuen Kwok-Yung, director of infectious diseases at the University of Hong Kong’s Department of Microbiology. The candidate vaccine is similar to a nasal spray vaccine that is already on the market and designed to start acting where respiratory viruses enter the body: the nose.
“Our concept is that we have flu coverage and Covid-19,” Yuen said in an interview.
Experimental fumigation studies have won the investment of the Coalition for Innovations in Epidemic Preparedness in Norway and the Hong Kong government, and will be enrolled in dozens of clinical trials around the world to identify effective vaccines to save you covid-19.
Last month, China began initial clinical studies of a nasal spray vaccine opposed to Covid-19, jointly developed by researchers from Xiamen University and the University of Hong Kong, as well as through vaccine maker Beijing Wantai Biological Pharmacy. Enterprise Co.
The Covid-19 double influenza vaccine is based on a temperature-adapted, weakened, replication-deficient influenza virus that only develops in the upper airways. Its developers used genetic engineering to remove the NS1 protein from the virus and insert the binding domain to the SARS-CoV-2 virus peak protein receptor, Yuen said. “And then we’ve shown very well that it works on animals,” he says.
In addition to being potentially protective against two viruses at once, the technique aims to stimulate an immune reaction at the site of the mucous membrane of the nose, Yuen said. Research by scientists at Imperial College London and published in the journal Science Friday indicates that a strong mucosal immune reaction is vital to helping other people breathe infections.
“A mucous membrane vaccine is a wonderful idea,” Yuen said.
A Chinese manufacturer will manufacture the experimental Phase 1 vaccine starting next month, he said. The trial will seek to demonstrate its protection and optimal dose. The results are expected a few months later.