The first order from an Indian dealer is substantially higher than expected, exceeding launch expectations with planned supplier follow-up orders in Singapore and Malaysia
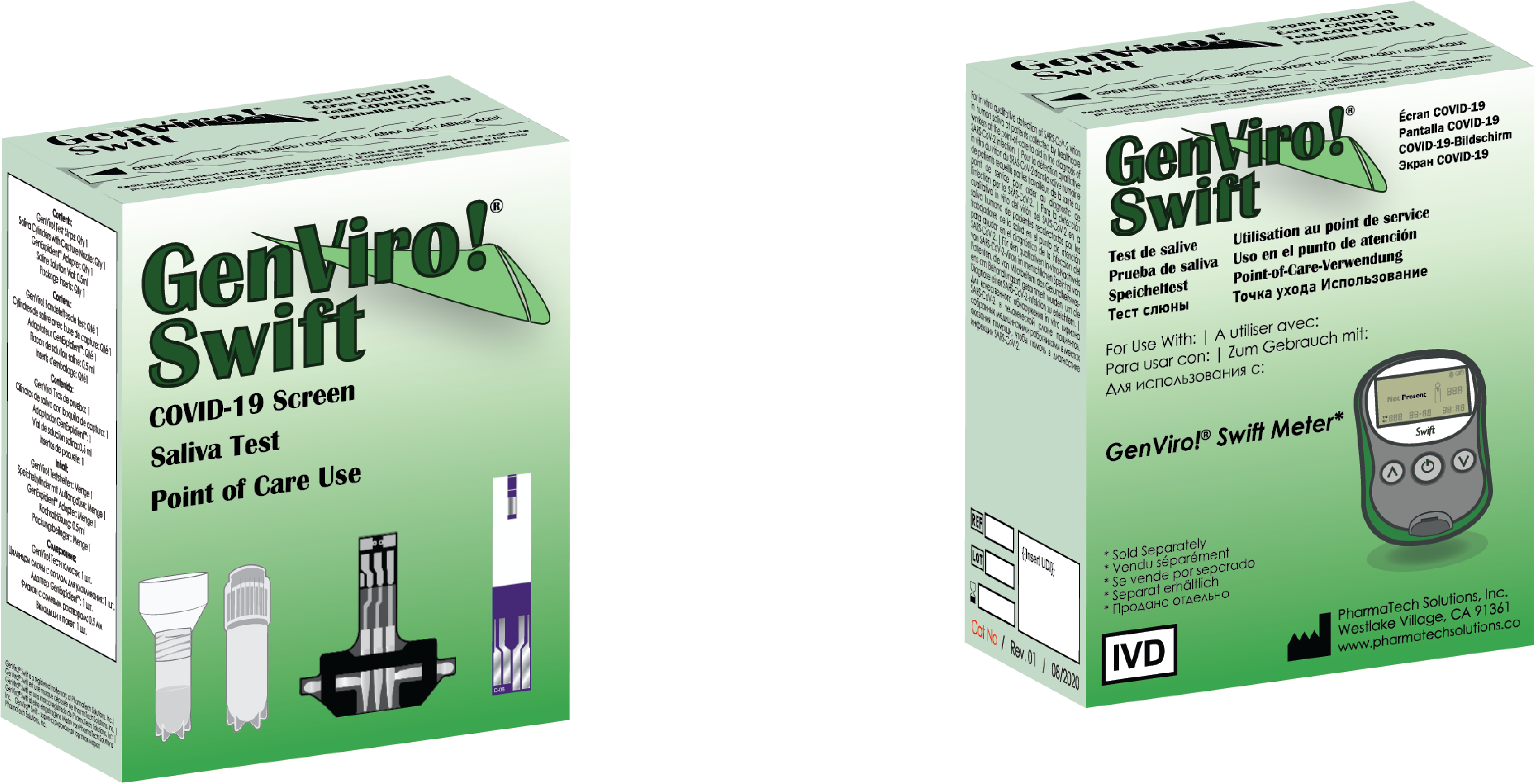
LOS ANGELES, CA / ACCESSWIRE / October 2, 2020 / Decision Diagnostics Corp. (OTC PINK: DECN) www. decisiondiagnostics. co, a leading manufacturer and global distributor of diabetic control strips designed to operate on legacy glucometers through its subsidiary Pharma Tech Solutions, Inc. , today demonstrated that it has won its first written order from an Indian distributor. The first of several expected follow-up requests is particularly higher than advertised and includes 500,000 GenViro!Saliva Swift Kits, 50,000 GenViro!Swift Verify Kits and 7500 GenViro! Swift Kit counters.
“Since the company reached the target date of September 26 to place our first reseller order, we are excited that you are exceeding what was expected based on our initial conversations with this dealer and dealer,” said CEO Keith Berman. continued efforts with this distributor and others, we are encouraged by your interest and, based on those discussions, we believe that we will get additional orders from Singapore and Malaysia in the coming weeks. We have global conversations that we will be reinforced by the fact that we have also signed an agreement with an American checking partner. “
In the past, the company had announced that GenViro kits!Covid-19 Saliva Swift conducted rigorous repeated checks at its production and product progression plant in Daegu, Korea. In addition, the company said it would put 3 retail products for foreigners. Professional use: the GenViro kit! Swift Saliva that adds 1 control strip, interface sleeve adapter, saliva collection container, thinner, packing inserts and will be priced at $8. 95 wholesale with an MSRP of $29. 95; GenViro Meter Kit!Swift will come with 1 meter for up to one hundred verification readings, a computer instruction video, which can also be viewed on the www. pharmatechdirect. com website, a reader’s user manual and a packing manual.
The GenViro! The Swift counter will be sold in bulk for $19. 95 with an MSRP of $39. 95 and will come with a user manual, packing note and DVD mentioned above containing the GenViro English instructional video!From the company, with subtitles in 4 other languages, Russian, German, French and Spanish with other adaptations of the video in Hindi and Bahasa melayu. Finally, the third product will be our GenViro! Swift Verify Kit to come with 1 check strip, an interface sleeve adapter and a packing manual. This kit is designed to provide a Covid-19 tracking check if desired. The Swift Verify kit will be priced $6. 95 wholesale with an MSRP of $24. 95.
The company’s new GenViro!™ products designed to verify Covid-19 are not yet available in the US. But it’s not the first time Or Puerto Rico, but emergency exemptions (U. S. ) are ongoing with the U. S. FDA. But it’s not the first time And more presentations will be made in the near future. The company also signed an agreement with an FDA-qualified check spouse and U. S. CLIA. But it’s not the first time To conduct verifications in accordance with existing FDA rules and continues to await FDA approval from our U. S. FDA.
On September 26, 2020, the company began launching its foreign package versions of its GenViro kits!Kits Saliva Swift and Swift Meter, plus it informs that your distributor for the Russian Federation will take over Genviro!And it will be responsible for the registration for emergency use approval in the Russian Federation.
SOURCE: Decision Diagnostics Corp.