They know that the country aspires to normality, which can lead to widespread use of a vaccine that immunicados most Americans opposed to COVID-19, but reminds us that a viable vaccine can come if there are effects of robust, verifiable and freely accessible studies. that show that it works and is helping without harm.
While efforts to create candidate vaccines have been heroic and enthusiastically followed by the public, in many tactics this has been the simple part, said Prakash Nagarkatti, immunologist and vice president of studies at the University of South Carolina.
“Vaccine generation is not confusing; any university that focuses on research has the generation to expand the vaccine,” he said. The challenge is to succeed over the challenge of clinical trials with a product that has proven to be effective.
By the time life can resume in the United States before COVID-19, USA TODAY has created an organization of experts in medicine, virology, immunology, logistics, and chain-of-origin disorders to estimate how close we are to getting a SARS-CoV-2 vaccine. , the virus that causes COVID-19.
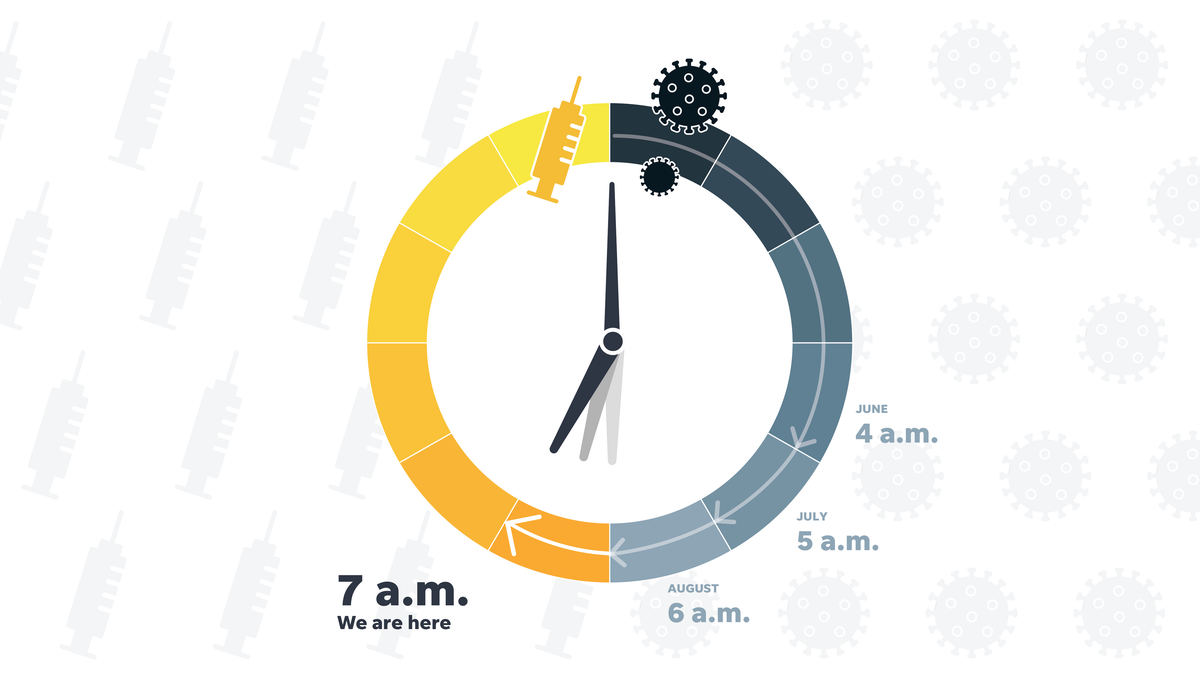
Each month, we ask the panelist to calculate where he believes the race is taking place and what time it is on the USA TODAY vaccination clock. will be given to anyone who needs it.
This month, for the fourth consecutive month, they are seeing steady progress, with the September clock reaching 7 a. m. It’s an hour closer to noon than last month, but still a little closer to the finish line.
”Amazing Complex’: What we know about how a vaccine will be distributed
On Wednesday, the Centers for Disease Control and Prevention published a manual describing how local public states and fitness systems plan and prepare for the release of one or more COVID-19 vaccines.
The CDC’s plan-making paper states that the vaccine would possibly be obtained in early November and that planners assume that until January 2021, “much more COVID-19 vaccine can also be available for distribution. “
In testifying before Congress later that day, CDC Director Robert Redfield said only limited amounts of vaccine would be available in the first place. The American public, he predicted, could not get it and “go back to general life” until next summer. Hours later, President Trump said Redfield had misrepresented the vaccine, ingesting more confusion about when there might be a vaccine on the market.
In any case, the U. S. Food and Drug Administration has not been able to do so. But it’s not the first time You will need to give your approval for any amount of vaccine to be distributed. USA TODAY panellists are investigating exactly how this will happen.
Dr. Stephen Hahn, director of the FDA, said there may be an intermediate endpoint, prior to the final touch of a 30,000-person trial, which can meet the criteria for so-called emergency use authorization. issued in connection with a federal fitness emergency.
Hahn said it could shorten the latest clinical trials of a candidate vaccine as long as there is enough knowledge to show that it is effective.
Our panelists said that fundamental data is just the beginning of what is needed.
At a minimum, knowledge will need to be brazenly communicated to the clinical network and thoroughly reviewed through the FDA’s External Advisory Committee on Vaccines and Related Biological Products, said William Schaffner, professor and expert on infectious diseases at Vanderbilt University School of Medicine in Nashville, Tennesse.
In addition, the FDA will not have to exert any political pressure to temporarily publish it, let’s say in time for the November 3 presidential election.
Thus, on 3 September, the Organization for Biotechnological Innovation issued a public letter reiterating the strict criteria to which its members adhere.
“Trusting science, and clinical procedure, is the way out of this pandemic,” dr. Michelle McMurry-Heath, president and CEO of BIO.
On 8 September, nine pharmaceutical corporations also issued a joint commitment to “high moral criteria and sound clinical principles” in progression and possible vaccines opposed to COVID-19.
“If we consider how the US government’s reaction to the US government has been a backlash, we will not be able to do so. But it’s not the first time To date it has fostered public mistrust, calculating dangers and benefits is becoming and early release is not a smart idea,” said Arti Rai, a professor of law and an expert in fitness law at Duke University. School.
Others, that intense care and reports of possible disorders make it less likely that a vaccine will be approved before it is fully monitored.
Dr. Paul Offit, director of the Children’s Hospital’s Center for Vaccine Education in Philadelphia, said he would accept as true with FDA officials if they feel a vaccine is in condition before clinical trials are completed.
“In fact, I don’t think these vaccines are going to be introduced until evidence has been falsified. I decide,” Offit said.
Dr. Otto Yang of the University of California, Los Angeles, sees no approval until the new year.
“I don’t think we can have a safety knowledge smart enough until at least winter (three to 4 months of knowledge verification). Shorter than that is very little time, no matter what effects they show,” said Yang, a medical professor and associate. head of infectious diseases at UCLA’s David Geffen School of Medicine.
The effectiveness of an experimental vaccine will have to be very high for early launch to be a moderate choice, said Dr. Gregory Poland, director of the Mayo Vaccine Research Group and editor-in-chief of Vaccine magazine.
The only cases where he sees a justification for early release are whether there have been primary adjustments to the death rate or headaches in others who have COVID-19, he said.
For some, however, public fear of protecting a vaccine perceived as hasty makes any fda-truncated approval procedure unacceptable. A low rate of adverse occasions, which can only occur with a full verification procedure, will have to come first, Dr. Monica Gandhi, professor of medicine and infectious disease specialist at the University of California, San Francisco.
“I know we’re desperate and eager to get out of this pandemic, but the most productive way to do that is to have an effective vaccine that is too, and for others to accept,” he said.
This month, the estimated deadlines through our experts to download a widely available vaccine ranged from five a. m. still dark and 10 a. m.
While the clock advanced by one hour, AstraZeneca Phase 3 transient closure last week injected a cautionary note into a more commonly positive chronological progression. News of the British player presenting a neurological condition consistent with a rare but severe inflammation of the spine. called transverse myelitis has pushed back some of our experts’ clocks.
But some, adding structural biologist Pamela Bjorkman, saw it as a sign.
“The encouraging thing is that AstraZeneca did the right thing and suspended the test until they understood what was going on,” said Bjorkman, who works at the California Institute of Technology. “This shows the importance of completing Phase 3 tests with a giant cohort of other people in the vaccinated and placebo groups, and also waiting long enough to see if there had been harmful side effects.
Dr. Kelly Moore, Associate Director of Immunization Education at the Immunization Action Coalition, added: “Pauses and research like this are an integral component of the clinical trial process. A hoop to cross in succession.
The vaccine editor, Poland, agreed: “Finally, a little common sense is to think among all the stakeholders that we want to stop, be careful and reflect our enthusiasm,” he said.
Panellists are also confident that Pfizer and Moderna, two of the other pioneers, have recorded vaccine trials almost entirely, Pfizer announced on September 12 that he plans to expand his vaccine trial to 44,000 volunteers to accompany 16- to 18-year-olds. like others with fitness disorders like HIV and hepatitis.
Moderna on Thursday published a 135-page plan describing how it conducts its Phase 3 clinical trials, the first of the primary vaccine to do so.
Learn about U. S. vaccines TODAY
The COVID-19 vaccine will start circulating 24 hours after approval, says federal government
Two-thirds of Americans say they might not get the vaccine when they first get it, according to a survey.
U. S. faces monkey shortage as call for COVID-19 research grows
While the United States is focusing on advancing clinical trials, the vaccine’s career may already be over in Russia and China, according to the clinical standards implemented.
In either country, coronavirus vaccines are now being given to the military and, in some cases, to the public. On Monday, the United Arab Emirates granted emergency use authorization to a COVID-19 vaccine developed in China for frontline fitness workers.
These vaccines have only completed what in the United States would be considered Phase 1 or Phase 2 clinical trials. Moving forward with the human use of these vaccines is “high-risk gambling,” Moore said.
“Without randomized controlled clinical trials, we would probably never fully perceive their true dangers or benefits,” he said.
Vaccines used in these countries have made headlines in the United States, but will be a component of American weapons, panellists said.
More data have been obtained on Chinese vaccines, 3 of which are recently found in Phase 3 clinical trials, said Sam Halabi, a professor of law at the University of Missouri who specializes in public aptitude law. For the Russian vaccine, very little knowledge is available.
“Neither country is known for its candor or transparency, so these vaccines are completely unknown. Obviously, the leaders of both countries have strong political motivations for claiming success,” UCLA’s Yang said.
None of the protocols in those countries were developed in consultation with the FDA, Schaffner said, and without US surveillance. A vaccine cannot be approved here.
“Russian and Chinese vaccines are characteristics of those countries, but also of the United States,” he said.
At the University of South Carolina, Nagarkatti also has other concerns: vaccines that oppose Chinese and Russian coronaviruses use a modified form of a bloodless virus called Ad5, and those vaccines are feared to not appear in others who already have antibodies to this. it is not an unusual bloodless virus or would possibly cause other forms of toxicity.
Not only that, he says. Previous Ad5 studies “have shown that this can make others more vulnerable to HIV/AIDS. “
Pamela Bjorkman, structural biologist at the California Institute of Technology
Dr. Monica Gandhi, infectious disease specialist at the University of California, San Francisco
Sam Halabi, Professor of Law, University of Missouri; researcher at Georgetown University’s O’Neill Institute for National and Global Health Law.
Florian Krammer, virologist at Icahn School of Medicine at Mount Sinai in New York
Dr. Michelle McMurry-Heath, president and ceo of the Biotech Innovation Organization
Dr. Kelly Moore, Associate Director of Immunization Education, Immunization Action Coalition; former member of the CDC’s Immunization Practice Advisory Committee; Chairman of the World Health Organization’s Immunization Practice Advisory Committee
Prakash Nagarkatti, immunologist and vice president of research, University of South Carolina
Dr. Paul Offit, Director, Center for Vaccine Education and EA in the Division of Infectious Diseases at Philadelphia Children’s Hospital
Dr Gregory Poland, Director, Mayo Clinic Vaccine Research Group, Editor-in-Chief, Vaccine
Arti Rai, professor of law and expert in health law at Duke University School of Law
Dr. William Schaffner, Professor of Preventive Medicine, Department of Health Policy and Professor of Medicine, Division of Infectious Diseases, Vanderbilt University
Prashant Yadav, Lead Researcher, Global Development Center, Medical Supply Chain Expert
Dr Otto Yang, professor of medicine and head of infectious diseases at UCLA’s David Geffen School of Medicine
The patient protection and fitness policy at USA TODAY is made imaginable in components through a grant from the Masimo Foundation for Ethics, Innovation and Competition in the Health Sector. The Masimo Foundation does not provide any editorial contribution.