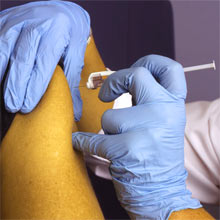
SACRAMENTO, Calif. – Click here to learn more about registration criteria and how to enroll UC Davis Health announced a partnership with Pfizer Inc. and BioNTech SE to participate in a global examination of an experimental vaccine opposed to COVID-19, adding a vital component of the university’s reaction to the fatal coronavirus pandemic.
As a component of this component, UC Davis Health plans to recruit up to two hundred component participants in the coming days for a clinical trial involving approximately 30,000 other people worldwide.
“We are excited to participate with our partners in this vital clinical trial for a COVID-19 vaccine,” said David Lubarsky, Vice-Chancellor of Human Health Sciences and Chief Executive Officer of UC Davis Health. “We are uniquely located to contribute to a potential breakthrough due to our experience in clinical trials, our ability to temporarily recruit for clinical trials, and our track record of raising awareness among minority communities.”
Ideal applicants for the COVID-19 vaccine exam are those who are at risk of being inflamed by the virus, such as physical care personnel and those operating in corporations with a large volume of customers, adding restaurants and retail stores. The essential staff who spend a lot of time away from home would also be an ideal candidate.
Applicants must be healthy and between 18 and 85 years of age. People from ethnic and racial teams who have been found to have been disproportionately affected by COVID-19, such as Latinos and blacks, are encouraged to participate.
UC Davis Health’s effort is led by principal investigator Timothy Albertson, president of UC Davis Health’s Department of Internal Medicine and senior expert on lung medicine and intensive care, and co-lead researcher Angela Haczku, associate dean of translational research.
The candidate vaccine has shown promising effects to date. other people around the world, ” said Albertson.
“Although there are several applicants for the COVID-19 vaccine in the world, it differs because it uses a new modified mNA (messenger ribonucleic acid), which includes the genetic code of SARS-CoV-2, the virus that causes COVID -19,” Haczku said. “We are very excited because this is the first time in history that mNR vaccines have been used against an infectious disease.”
The study trial, known as the Phase 2/3 study, aims at the efficacy and appearance effects of a non-married nucleoaspect modified messenger RNA candidate (modRNA) of its BNT162 mRNA-based vaccine program. The candidate vaccine has been rigorously evaluated in the United States and Germany and has already shown significant positive results.
UC Davis has been an active response to coronavirus disease since UC Davis Medical Center providers diagnosed and treated the first obvious case of COVID-19 in the U.S. acquired through the network in February.
UC Davis’ existing efforts involve extensive collaboration between pneumologists and infectious disease specialists in the comprehensive medical center care sets, clinical laboratory pathologists, medical microbiology virologists, the California National Primate Research Center, and the Center for Immunology and Infectious Diseases. The university seeks to have a greater perception of the biology and infectious pathology of this new virus and collaborate in new approaches of remedy and diagnosis.
Pharmaceutical giant Pfizer, which has partnered with Germany’s smallest BioNTech, has chosen vaccine sites known for its expertise in world-class studies, infrastructure and concentrations close to known and expected positive cases of COVID-19.
“This upcoming COVID-19 vaccine clinical trial is a historic opportunity for UC Davis School of Medicine and our network to directly contribute to preventing the spread of COVID-19 and potentially saving lives around the world.” said Allison Brashear, dean of UC Davis Medical School. “Our university medical center is well-provided and staffed to play this important role and we look forward to playing an active role in vaccine development.”
UC Davis Health serves a geographic domain populated across races and ethnicities, expanding the possibilities of identifying clinical trial applicants with more varied backgrounds than other communities in the United States. The pandemic has been especially complicated for blacks and Latinos who are inflamed and die at a higher rate than other groups.
“As we have demonstrated this pandemic, our role as the only educational medical center in the region is to promote science and the percentage of what we notice for the sake of greater public fitness,” said Lubarsky, executive director of UC Davis Health. “By doing so, we can help eliminate fitness disparities in underrepresented communities when new remedies and remedies emerge.”
Pfizer and BioNTech announced their access to the Phase 2/3 complex on July 27, when they enrolled the first organization of 120 sites that pledged to recruit participants who threatened to contract the virus, known on the clinical network as SARS-CoV-2.
The Phase 2/3 trial is designed as a 1:1 experimental vaccine candidate for a placebo, a randomized blind through the observer to discharge the safety, immune reaction and efficacy knowledge needed for regulatory review.
The main purpose of the trial is to save COVID-19 in others who were not inflamed with SARS-CoV-2 prior to vaccination and to save COVID-19, whether participants have already been inflamed with SARS-CoV- or not. 2. A secondary objective is to avoid coVID-19 bass in these groups.
If the vaccine candidate’s good luck continues, Pfizer and BioNTech have said they are on track to request a regulatory review starting in October. If regulatory approval or approval is obtained, corporations plan to supply up to one hundred million doses by the end of 2020 and approximately 1.3 billion doses by the end of 2021.