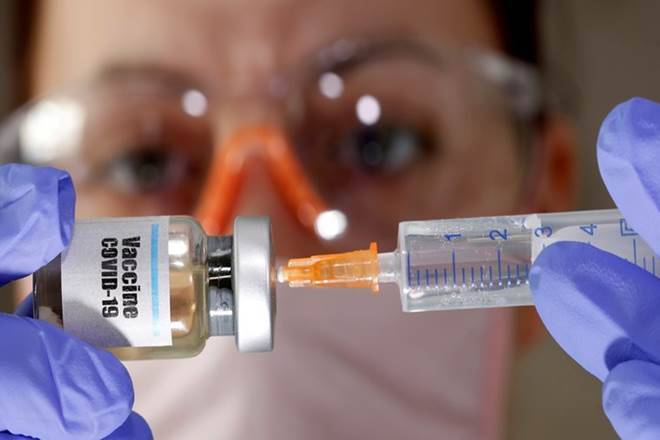
A COVID-19 candidate vaccine jointly developed by pharmaceutical giant Pfizer and German biotechnology company BioNTech induces a “robust” immune reaction in healthy adults aged 18 to 55, according to an interim report from an early-stage clinical trial, published in the journal Nature. Wednesday.
Researchers noted that BNT162b1 is an RNA vaccine that triggers an immune reaction by mimicking the mRNA molecule used through the new SARS-CoV-2 coronavirus to build its infectious proteins.
According to the study, the candidate vaccine is administered intramuscularly and in human cells to produce proteins that are components of the SARS-CoV-2 receptor binding domain, as opposed to the immune formula being trained to produce antibodies.
They stated that these vaccines are sometimes thought to have facilitated the immediate progression of SARS-CoV-2 vaccines. They said he was one of many applicants for RNA vaccines that were being studied in parallel to determine their variety in order to move on to a test of their type and efficacy.
In the ongoing phase 1/2 trial, scientists evaluated the type, side effects and dose of the candidate vaccine in forty-five healthy adults (23 men and 22 non-pregnant women) aged 18 to 55 years. Participants were randomly assigned to obtain 10 micrograms (g), 30 go hundred g of BNT162b1, or a placebo, the study noted.
Participants in the 10 gy 30 g teams also won a momently dose on the 21st, the scientists said. higher with the dose, within seven days of vaccination, adding injection site pain, fatigue, headaches, fever and sleep disorders.
They claimed that the vaccine triggered a “solid immune response” in the participants, which was accumulated with the point of dose and with an injection for the time being. According to the study, antibodies opposed to SARS-CoV-2 were provided 21 days after a singles vaccination at all dose points, and there was a really extensive accumulation of antibodies neutralizing SARS-CoV-2 seven days after the 10-go 10-go dose is administered.
He noted that the immune reaction is much more potent in the 30g organization than in the 10g organization, however, scientists said there were no noticeable differences in the immune reaction between 30 gy 100 g teams after a dose, and as participants who won 100 g. The dosage also experienced major side effects. They didn’t get a momentary dose.
Participants’ neutralizing antibody grades were reported to be 1.9 to 4.6 times higher than those of patients recovering from SARS-CoV-2 infection.
Although this comparison provides a baseline for comparing vaccine-induced immune reaction and the possibility of the vaccine providing protection, the researchers say that Phase 3 trials are needed to determine the efficacy of BNT162b1.
They said they also recruited adults between the ages of 65 and 85, and added that later stages would prioritize the registration of more varied populations.
Get live equity costs from BSE, NSE, U.S. market. And the latest liquidity value, mutual fund portfolio, calculate your taxes, the source of the income tax calculator, meet the most productive winners on the market, the most productive losers and the most productive equity funds. Like it on Facebook and stay with us on Twitter.
Financial Express is now on Telegram. Click here to subscribe to our channel and stay up to date with the latest News and Updates from Biz.