Islamabad: The National Institute of Health of Pakistan (NIH) obtained official approval from the drug regulatory authority to conduct 3 clinical trials of a COVID-19 vaccine in the country.
The Pakistan Pharmaceutical Regulatory Authority (DRAP) approved the trial of the vaccine developed through CanSinoBio, a vaccine developer in China, and the Beijing Institute of Biotechnology.
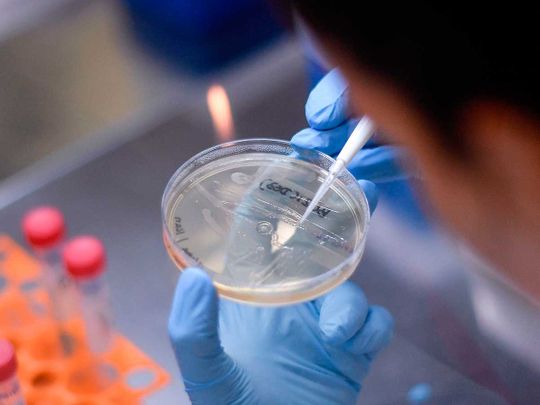
The first trial in Pakistan is likely to be held at the Indus Hospital in Karachi, for which 200 volunteers, men and women, have been enrolled and will last for 56 days, in which 3 inactivated virus injections will be given to volunteers.
Nearly 93% of other people inflamed by coronavirus in Pakistan have recovered.
Clinical trials to be conducted in Pakistan
Pakistan’s NIH and AJM Pharma are working with CanSinoBio to verify a possible vaccine opposite COVID-19, known as the five adenovirus vector of the recombinant vaccine opposed to the new coronavirus (Adfive-nCoV).Aamer Ikram, executive director of niH, would be the lead researcher of the clinical trial.
The trials would be carried out at the most sensible fitness institutes in the country, in addition to the Aga Khan University of Medicine and the Indus Hospital of Karachi, the Shaukat Khanum Memorial Hospital and the Lahore University of Health Sciences and International Hospital. Shifa in Islamabad. National Bioethics Committee of the Pakistan Health Research Council.
Paid clinical trials would recruit others of other ages, genders and ethnicities, as a more varied examined population is helping to ensure some protection and effectiveness.The global clinical network will largely monitor the progress of the clinical trial, authorities said.improve the availability of vaccines at moderate costs in Pakistan and also the country’s ability to expand vaccines locally, according to the statement.
China leads vaccine race
CanSino Biologics is China’s first COVID-19 vaccine patent to conduct phase 3 trials in several countries in Saudi Arabia, Russia, Brazil and Chile.CanSino the first company in the world to transfer to human trials in March.At the same time, Sinopharm Sinopharm, a Chinese state-owned company, collaborates with the International Center for Chemical and Biological Sciences at Karachi University in Pakistan to deliver coronavirus vaccines in Pakistan.Sinopharm has also introduced phase three clinical trials of the COVID-19 vaccine in Abu Dhabi, United Arab Emirates, with up to 15,000 volunteers.
Progression and stages of the vaccine
Exploratory step
Preclinical stadium
Clinical development
Regulatory and approval
Manufacturing
Quality control
Phases of the clinical trial
Clinical is a three-phase process:
Phase one: During phase one, only a few people get the control vaccine to verify safety.
Phase two: In the moment phase, the vaccine is given to several hundred people in other groups, for example through age and physical health.
Phase 3: The test is extended to phase 3 and the vaccine is given to thousands of people and tested to determine its efficacy and safety.
Phase 4 trials are voluntary studies that can be conducted after a vaccine is approved and published for increased safety, efficacy, and use.
Dear reader,
This segment is about life in the United Arab Emirates and data you cannot live without.
Sign up to read and complete gulfnews.com