GET THE APP
COVID-19 Vidyagama Standby Program
A small mistake can cause a crisis in Dasara’s celebrations
Director Vijay Reddy no longer
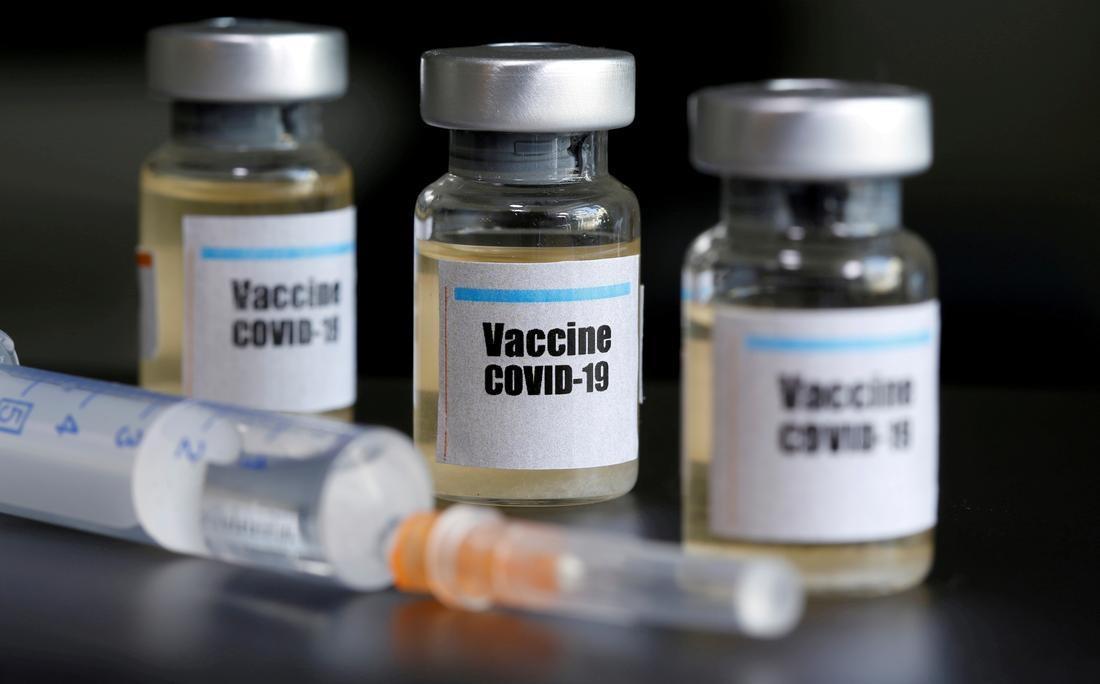
Bharat Biotech asked to present complete ph 2 knowledge of its COVID-19 vaccine prior to the ph 3 trial
Covid-19: India 73272 new cases, 926 new deaths
Update of K’taka COVID-19: 10913 new cases, 9091 releases, 114 deaths on October 8
Bharat Biotech asked to present complete ph 2 knowledge of its COVID-19 vaccine prior to the ph 3 trial
Rahul Gandhi attacks the opposite environment of bulletproof cars for soldiers
Army thwarts Pak to push guns into Kashmir and recovers 4 rifles
Srikant Datar, born in India, appointed dean of Harvard Business School
Israel to bring ‘immediately’ 2,000 Ethiopian Jews
Armenia and Azerbaijan agree on ceasefire in Nagorno-Karabakh
SFI launches cross video to highlight key SOP when pools reopen
We think it’s an underrated score, but the port behaved differently: Iyer
Number 1 Djokovic escapes in 5, will face No. 2 Nadal in final
ONGC to merge between HPCL and MRPL after June 2021
RBI makes RTGS 24×7 from December
GDP is likely to contract by 9. 5% in fiscal year 21: RBI
It’s intimidating: Kristen Stewart on the role of Princess Diana in a biographical film
‘Jai Ho’ actress Sana Khan leaves the films and says she’s ‘at the service of humanity’
Amitabh Bachchan joins Deepika and Prabha’s multilingual sci-fi film
Rosogolla Biryani’s viral video leaves internet users outraged; Would you like to check it out?
Who is Jackson Oswalt, Tennessee Teen, the youngest user to carry out nuclear fusion?
Who is Yatin Oza and why did the Gujarat High Court convict him of contempt?
Hero MotoCorp launches 24-year road × 7 for its customers
Nissan to present Magnite to global on October 21
New Delhi: Bharat Biotech, which had requested DCGI APPROVAL for phase 3 clinical trials of its COVID-19 candidate vaccine, requested to submit comprehensive knowledge on the protection and immunogenicity of the ongoing Phase 2 trial, as well as provide some clarification, before moving on to the next step.
The candidate vaccine, “Covaxin”, is developed through Bharat Biotech in collaboration with the Medical Research Council of India (ICMR).
According to officials, Hyderabad-based vaccine manufacturer filed an application with the Indian Drug Comptroller-General (DCGI) on 2 October, requesting permission for a randomized, double-blind, placebo-controlled multicenter trial of its COVID-19 candidate vaccine.
The firm’s request said the canopy would cover 28,500 subjects over the age of 18 and be held at 19 sites, including Delhi, Mumbai, Patna and Lucknow, in 10 states.
According to sources, Covaxin Phase 2 trial is underway and the dose has not yet been administered to volunteers at some sites.
“The company presented the Phase 3 clinical trial protocol as an intermediate knowledge of phase 1 and 2 clinical trials,” one official said.
The Committee of Experts on The Subject (SEC) of the Central Organization for the Control of Drug Standards (CDSCO) discussed the application on 5 October.
“After careful deliberation, the Committee found that the design of Phase 3 was initially satisfactory, with the exception of an explanation of the definition of asymptomatic, etc.
“However, the test should be initiated with a known appropriate dose of Immunogenicity Knowledge and Phase 2 Protection. As a result, the company will need to submit knowledge of immunogenicity and protection of the Phase 2 trial for review,” the panel said in its recommendations.
During its discussion, the SEC also noted that the vaccine was well tolerated in all dose equipment and that no serious adverse occasions have been reported to date, one source said.
The maximum non-unusual adverse pain at the injection site, which was transiently resolved, the source said.
The Phase 3 test application proposed a dose of 0. 5 ml on days 0 and 28, Resources said.
In addition, Bharat Biotech, a candidate vaccine developed through Zydus Cadila Ltd, is found in phase 2 human clinical trials.
The Pune-based Indian Serum Institute, which partnered with AstraZeneca to manufacture the Oxford COVID-19 vaccine candidate, is also conducting 2 and 3 human clinical trials with the candidate in India.
GET THE APP
Looks like you’ve activated an ad blocker. To continue reading, turn off or whitelist Udayavani.