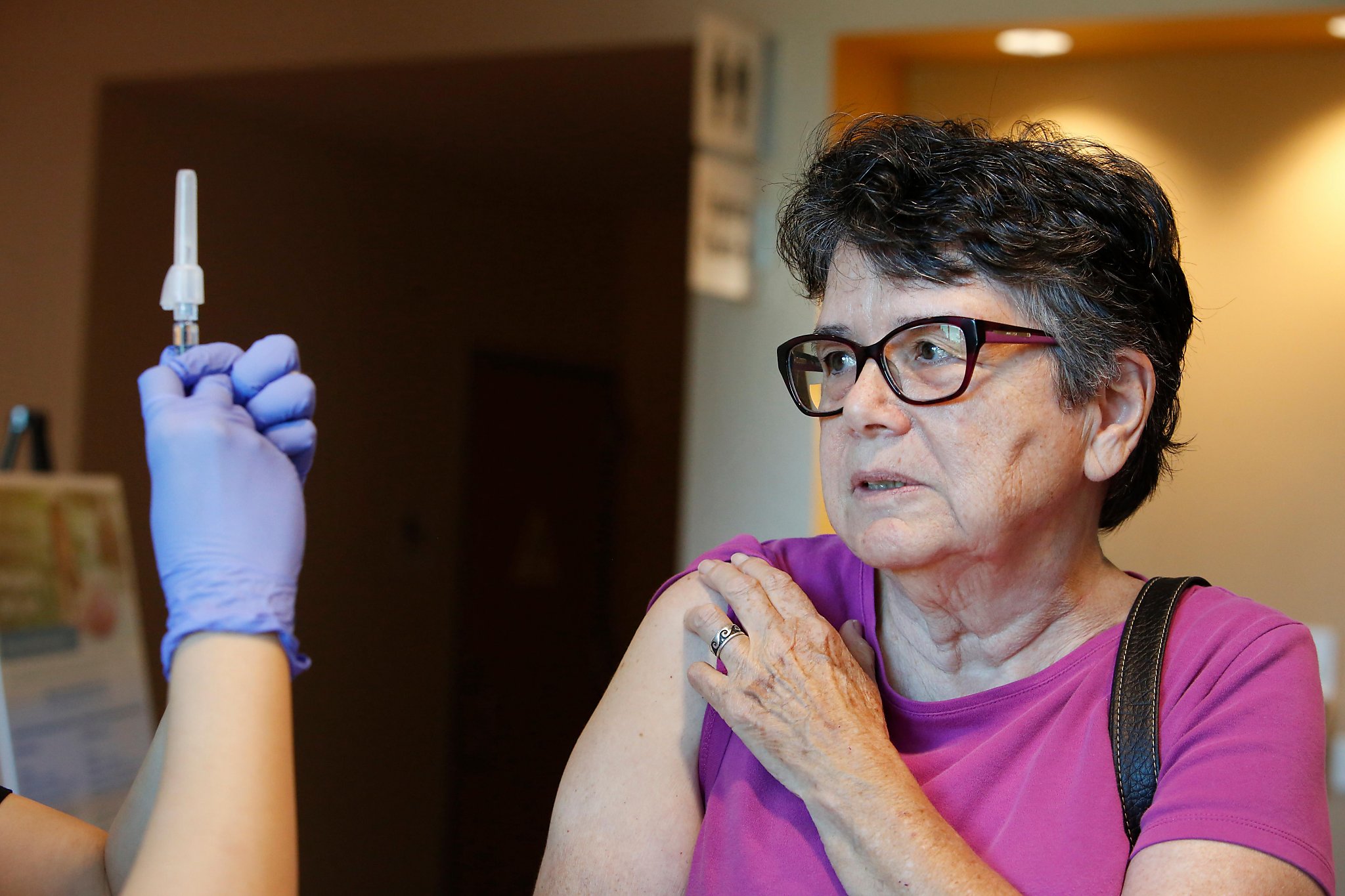
Kaiser Permanente in California and Oregon, adding sites in Santa Clara and Sacramento counties, has joined a global test of one of the first coronavirus vaccines to start large-scale human testing, the physical care provider announced Wednesday.
The Phase 3 trial, the last step before federal approval, is the first to look for Bay Area volunteers in the dramatic foreign race for a coronavirus vaccine.Kaiser delivered the vaccine to his first volunteers in Northern California on Monday.
Approximately 1,400 participants at 4 Kaiser sites will get the vaccine developed through Pfizer Inc.and the German generation company BioNTech, which are sponsoring the trial. Developers plan to enroll another 30,000 people at more than 120 sites worldwide.
The vaccine uses artificial genetic material, called messenger RNA or mRNA, to induce the body’s immune formula to expand cells and antibodies to attack the virus.This is a new vaccination technique that has still been used in approved vaccines.
“In some respects (the trial) is very similar to the type of vaccine trials we do all the time.What’s not typical is the speed at which it all happened,” dr.Nicola Klein, director of the Kaiser Permanente Center for Vaccine Studies.and principal investigator of the Northern California trial.” As a rule, we will care about brands over the next few years.”
Kaiser’s component will be controlled through Kaiser Permanente’s Research Division in Oakland, the Department of Research and Evaluation in Pasadena, and the Center for Health Research in Portland, Oregon.
Participants must be adults between the age of 18 and 85 who are members of Kaiser.They can’t be pregnant or plan to be pregnant. Kaiser is looking for volunteers who are more likely to expand COVID-19, adding older people with underlying physical fitness disorders or doing jobs where they are most at risk of exposure to the virus.
The trial is a double-blind placebo-controlled study, in which one part of the participants will get the vaccine and the other part a placebo.Neither participants nor researchers will know who won the vaccine.
The global trial was introduced at the end of July, at approximately the same time as another primary trial of a vaccine developed through Moderna.The Modern vaccine also uses mRNA technology.
Pfizer and Moderna have secured the main investment of the U.S. Health and Human Services Agency.As a component of President Trump’s Warp Speed operation to temporarily launch an effective vaccine.Pfizer has received up to $1.95 billion for the manufacture and distribution of approximately one hundred million doses of its vaccine. Modern earned approximately $955 million.
Three vaccines, manufactured through Johnson
Erin Allday is editor of the San Francisco Chronicle.Email: [email protected]
Erin Allday is a fitness journalist who writes about infectious diseases, stem cells, neuroscience and customer fitness topics, such as fitness and nutrition.He has been at the speed of fitness since 2006 (minus a nine-month period in Mayor Gavin Newsom’s ward Before joining The Chronicle, Erin worked in Bay Area newspapers and covered a little of everything, adding business and technology, municipal government and education.He was part of a team of reporters who won a Polk Regional Reporting Award in 2005 for a series of articles on outsourcing tasks from Santa Rosa to Penang, Malaysia.Erin began his career as a journalist at the Daily Californian student newspaper and many years later, Berkeley is still home.