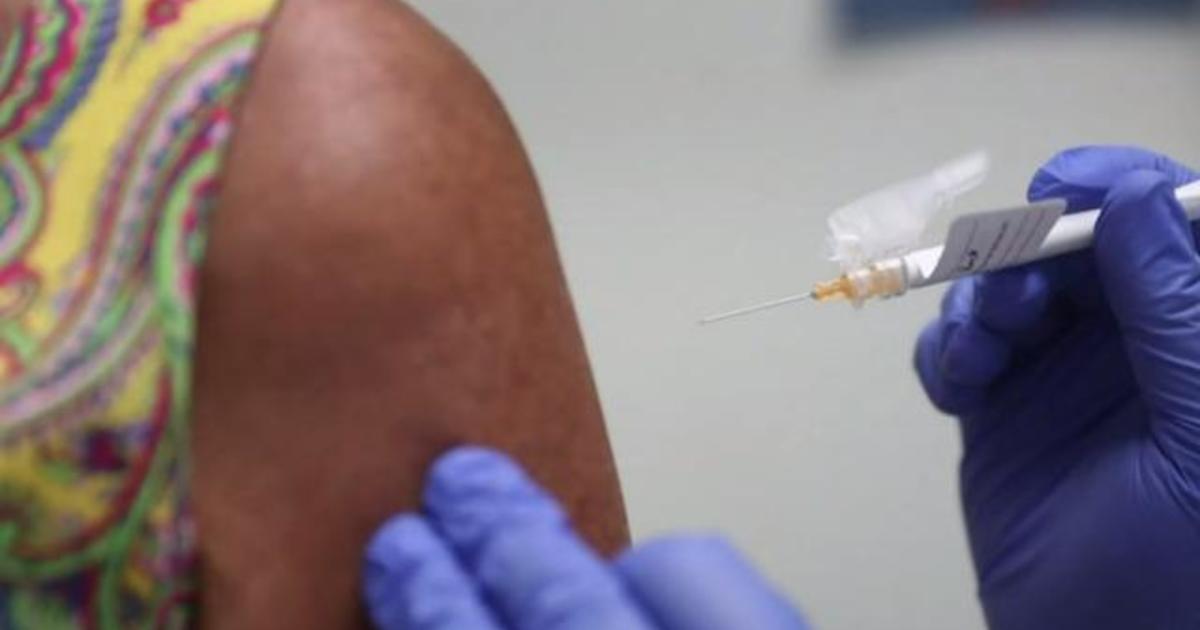
A long-awaited coronavirus vaccine may be available in two months, according to drug manufacturer AstraZeneca, who is preparing treatment.
The Anglo-Swedish drug manufacturer is working with the University of Oxford to expand one of the maximum monitored COVID-19 vaccines, which is found in expired trials in the United States, the United Kingdom and other countries for their protection and efficacy. regulators will have to approve the vaccine for widespread use.
AstraZeneca said he will analyze knowledge of his vaccine trials in November and December and, while the effects seem promising, he will act temporarily to boost production and get government approval in the United States and elsewhere, Executive Chairman Pascal Soriot said Thursday.
“We will be able to obtain tons of millions of doses of international vaccines until January,” Soriot said at a call for the convention on effects on Thursday.
AstraZeneca and Oxford University have been a vaccine in the United States for weeks. Trials were stopped in September after a player had adverse reactions. AstraZeneca and Oxford resumed last month.
AstraZeneca and Oxford are the leading applicants in the race to expand an effective COVID-19 vaccine. The university has also conducted phase 3 trials in Brazil and South Africa.
Other companies, such as Moderna and Pfizer, are also conducting vaccine trials. Moderna said his vaccine would not be in a position to be widely used until next spring. Pfizer said he could request emergency use of his vaccine no later than this month. If this is the case, a imaginable vaccine would reach the United States, while the country has an absolute record of COVID-19 cases.
According to johns Hopkins University’s knowledge, coronavirus instances in the United States have increased by 45% in the past two weeks, reaching an average of 86352 in 7 days. COVID-related hospitalizations are also setting records across the country.
In addition to the vaccine, AstraZeneca said they were preparing a “long-acting antibody” remedy that they said would strengthen the immune formula and protect vulnerable populations from prolonged COVID-19 outbreaks. The remedy would protect for six months or a year. depending on the dose, the corporate reported.
“Whether positive or negative, it doesn’t matter, everyone understands it,” AstraZeneca’s executive vice president Menelas Pangalos said at the convention call. “Imagine a care home [establishment] with an infection. You can come to the care home and let them know protection. “
AstraZeneca and Oxford are committed to offering their COVID-19 vaccine with no benefits for the pandemic. AstraZeneca will continue to supply the vaccine to the countries to come with no benefit after controlling the pandemic, and rich countries will pay a “relatively low cost,” Ruud Dobber, president of the company’s U. S. unit, told The Associated Press.
Originally, AstraZeneca expected to deliver millions of vaccines until September. The delay in vaccine progression is partly due to the decrease in COVID-19 instances before this year, slowing the progress of human trials that depend on subjects naturally exposed to the disease. Soriot said.
AstraZeneca updated its vaccine on the same day it published its third trimester results. Revenue increased by 3% to $6. 5 billion in the quarter and earnings decreased by 1% to $1. 8 billion.
The Associated Press contributed to the report.
Quotes with delay of at least 15 minutes.
Market knowledge through ICE Data Services ICE Limitations Driven and implemented through FactSet News through The Associated Press Legal Statement.