A leading company oriented towards virtual transformation.
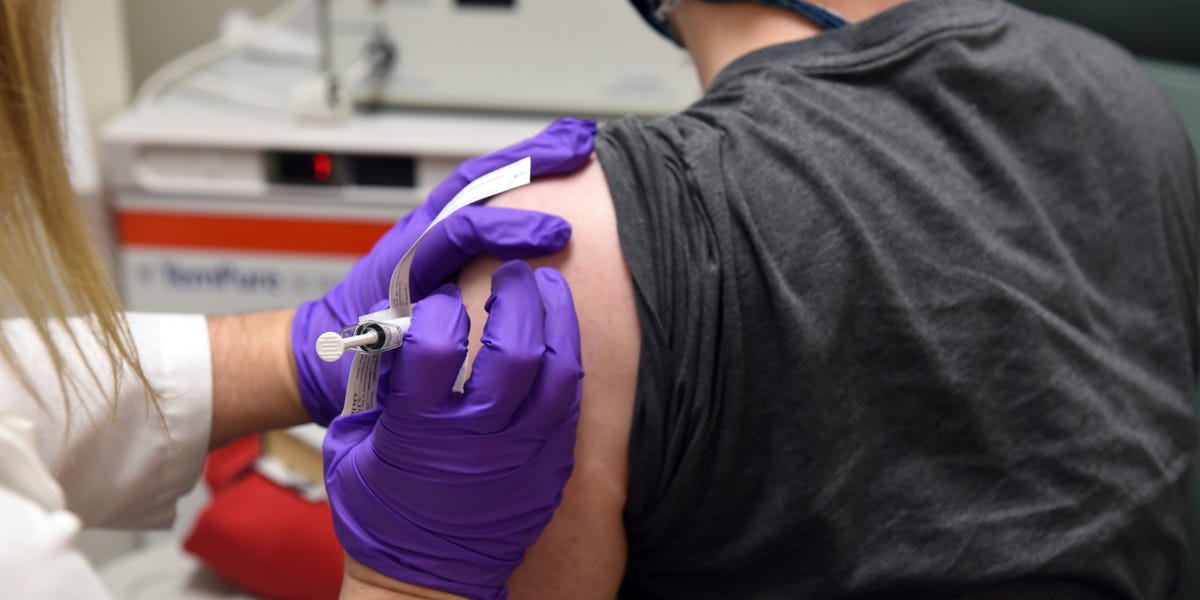
A large phase 3 that tests a COVID-19 vaccine that is being developed through AstraZeneca and Oxford University at dozens of sites in the US. But it’s not the first time It has been suspended due to a suspected serious adverse reaction in a player in the UK.
A spokesman for AstraZeneca, a pioneer in the race for a COVID-19 vaccine, said in a statement that “the company’s popular review procedure has caused a pause in vaccination to allow for review of safety knowledge. “
It was not without transparent delay who had suspended the trial, although it is imaginable that it was voluntarily placed through AstraZeneca and was not ordered through a regulatory agency. The nature of the adverse reaction and when it happened were also not known without delay, although it is believed that the player is recovering, according to a user familiar with the case.
The spokesman described the pause as “a regime action that will have to happen whenever there is a prospectively unexplained disease in one of the trials, while it is being investigated, making sure that we maintain the integrity of the trials. “The spokesman also said the company is “working to speed up the review of the singles occasion in order to minimize any possible effects on the test schedule. “
A person familiar with the progression said the researchers had been informed that the trial had been suspended as a “great precaution. ” A user familiar with the matter, who also spoke on condition of anonymity, said the discovery was having an effect. about other ongoing AstraZeneca vaccine trials, as well as clinical trials conducted through other vaccine manufacturers.
Clinical shutdowns are unusual and it is known how long AstraZeneca can last. But the progress of the company’s test, and that of all COVID-19 vaccines in progression, is being largely observed given the urgent need for new tactics to curb the global pandemic. Lately there are nine candidate vaccines in phase 3 trials. AstraZeneca is the first phase 3 COVID-19 vaccine trial to be discontinued.
Researchers conducting other tests are now on similar cases of adverse occasions through navigation knowledge bases reviewed through a so-called knowledge monitoring and protection committee, the user said for the time being.
AstraZeneca did not begin its Phase 3 test in the United States until the end of August. The US essay is a us-in-the-world test. But it’s not the first time It is recently underway at 62 sites across the country, according to Clinicaltrials. gov, a government record, some have not yet begun recruiting participants. Phase 2/3 trials have already been introduced in the UNITED Kingdom, Brazil and South Africa.
There are a number of other reactions that can be considered serious suspicious side effects, symptoms requiring hospitalization, life-threatening illness, and even death. It was also not immediately transparent in which clinical trial the appearance effect had occurred, a transparent option is the ongoing phase 2/3 trial in the UK.
While it is not yet clear how serious and uncommon the adverse occasion may be, location may have an effect on the temporary availability of the UK trial’s knowledge of effectiveness. This knowledge is considered an integral component of any application to off download emergency use authorization for the U. S. Food and Drug Administration vaccine, and may undermine President Trump’s efforts to accelerate implementation. vaccine before the November election.
A phase 1/2 exam published in July reported that approximately 60% of the 1,000 participants who won the vaccine experienced side effects. All adverse events, including fever, headache, muscle aches and injection site reactions, were considered mild. or moderate All reported appearance effects also subaspected the test.
The vaccine, known as AZD1222, uses an adenovirus that carries a gene for one of the proteins in SARS-Cov-2, the virus that causes COVID-19. Adenovirus is designed to induce the immune formula to generate a protective reaction opposed to SARS-2. The platform has been used in an approved vaccine, but has been tested in experimental vaccines against other viruses, adding Ebola.
The Phase 3 trial in the United States aims to recruit some 30,000 participants to 80 sites across the country, according to a statement released last week through the National Institutes of Health.
It was not clear without delay what measures were being taken at the sites examined throughout the United States in response to the suspension. Clinical interruptions in ongoing studies involve a pause in the recruitment of new participants and the dosing of existing participants, unless considered in the most productive interests of the protection of participants continue with the dosage.
In AstraZeneca’s statement, the corporate spokesman noted that “in giant trials, the disease occurs by chance, but will need to be reviewed independently to determine this carefully. “The spokesman also said that the corporation was “committed to protecting our participants and the highest criteria for conducting our trials. “
Get the latest research on the economic and advertising effect of Business Insider Intelligence coronaviruses on how COVID-19 affects industries.