“r.itemList.length” “this.config.text.ariaShown”
“This.config.text.ariaFermé”
AstraZeneca announced that she has begun recruiting adults for a Phase 3 trial in the United States of her candidate vaccine opposed to Covid-19 AZD1222.
AstraZeneca (AZN) said that the trial, which will feature 30,000 participants over the age of 18, will compare the safety, efficacy and immunogenicity of AZD1222 for the prevention of Covid-19.
The U.S. trial, D8110C00001, is funded by the Advanced Biomedical Development Authority (BARDA) and the National Institute of Allergy and Infectious Diseases (NIAID), and led by AstraZeneca.
“We are very pleased that AZD1222 has demonstrated its protection and immunogenicity in all adult teams and we are proud to work with BARDA and NIAID to drive the progression of this vaccine,” said Mene Pangalos of AstraZeneca.”If clinical trials show that the vaccine protects against COVID-19 disease and is approved for use, we will work hard to make it international as fair and equitably as possible.”
Trial participants will get two doses of AZD1222 or saline 4 weeks apart, twice as likely as the saline vaccine.3,000 participants.
AstraZeneca added that the clinical progression of AZD1222 is progressing globally with complex clinical trials underway in the UK, Brazil and South Africa and trials are expected to begin in Japan and Russia.
These trials, along with the US Phase III clinical trial, have been conducted in the united states.Recruit up to 50,000 participants worldwide. Complex trial results are expected later this year on the infection rate in clinical trial communities.
In July, AstraZeneca reported that the provisional effects of the ongoing Phase I/II COV001 trial showed that AZD1222 tolerated and generated physically potent immune responses opposed to SARS-CoV-2 in all participants evaluated.
Shares of AZN have gained 12% this year, as the drug maker joined the list of corporations interested in introducing a possible coronavirus vaccine. 12 months.
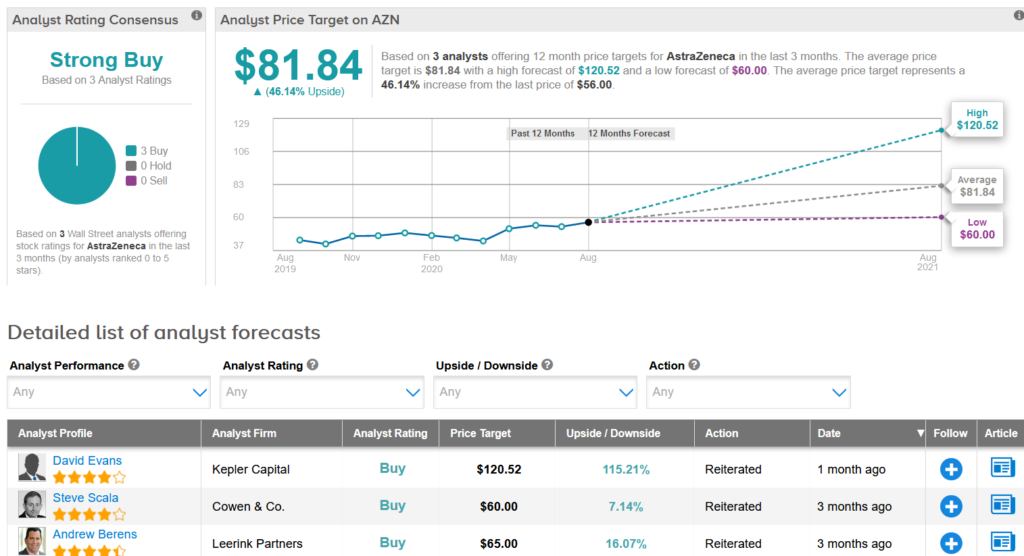
In general, inventory achieves a strong buying consensus from the analyst network on 3 unanimous purchase odds (see AstraZeneca inventory research on TipRanks).
Related News: T2 Bioystems increases 19% in FDA approval for Modern Covid-19 molecular test in talks to supply 40 million doses of his Covid-19 vaccine to Japan AstraZeneca rises in a report that Trump could accelerate the Covid-19 vaccine candidate
Tuesday before marketing: here’s what you want to know before the market opens
Sanofi claims that the drug Kevzara meets the evaluation criteria of the Covid-19 study
Apple asks vendors to build 75 million iPhone 5Gs: report
Amazon gets smooth FAA for Prime Air drone delivery