“R.itemList.length” “- this.config.text.ariaShown
“This.config.text.ariaFermé”
Arcturus Therapeutics announced that it has reached a final agreement with the Israeli Ministry of Health for the vaccination of its vaccine candidate Covid-19.
Arcturus Therapeutics (ARCT) stated that, under the terms of the agreement, the delivery to Israel of the doses of its LUNAR-COV19 mRNA candidate vaccine, also known as ARCT-021, is conditioned on the final touch of clinical and short-term trials. Regulatory steps. Financial terms were released. Shares rose 6.6 percent to close at $62.65 on Friday.
Arcturus, in San Diego, is a clinical-stage company targeting ribonucleic acid (RNA) drugs and vaccines and has developed an internal messenger RNA platform. He specializes in the discovery, progression and marketing of curative products for rare diseases and vaccines.
“We are pleased to have signed the final agreement of origin with the Israeli Ministry of Health. Arcturus is revered for playing a key role in Israel’s COVID-19 immunization strategy,” said Arcturus CEO Joseph Payne. “We appreciate the commitment of the Israeli Ministry of Health to our mNR differentiating candidate vaccine and look forward to advancing the progression of ARCT-021.”
Israel is the country at the moment, in addition to Singapore, to protect the source of the ARCT-021 vaccine. Arcturus said it was in active discussions with other governments in key markets and in other parts of the world. In collaboration with its production partners, Arcturus is in the process of producing millions of doses by 2020 and is positioned to obtain loads of millions of doses consistent with the following year, the company added.
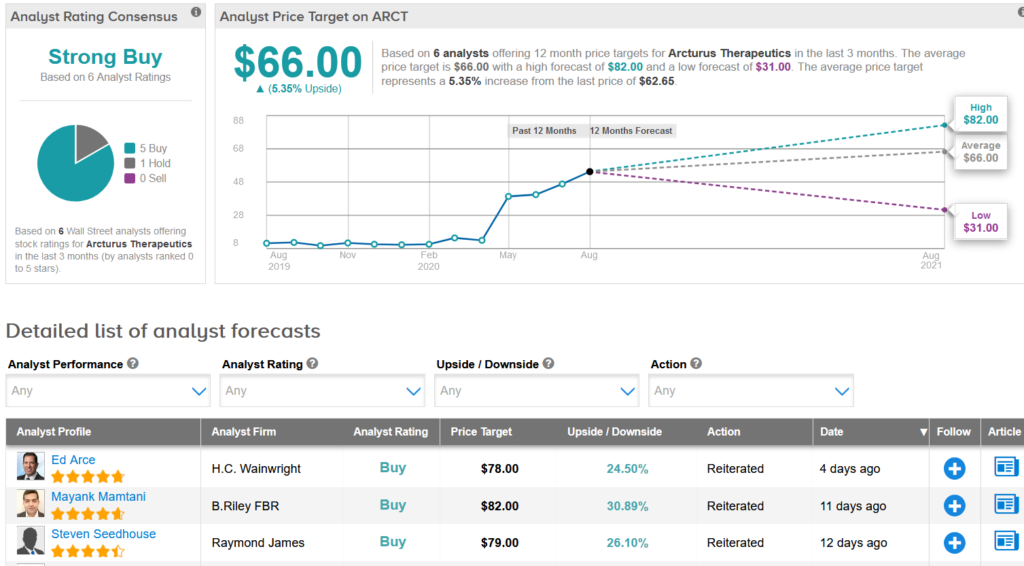
Arcturus shares have risen 27% since the company announced on August 11 that it had begun measuring its covid-19 vaccine candidate in a 1/2 phase study. (See Arcturus action research in TipRanks)
H.c. Wainwright analyst Ed Arce reiterated a buying note to rent in inventory with a target of $78 (potential for a 25% increase), saying he expects the agreement with Israel to be an indicator of other governments acquiring deals in the coming months. .
“LUNAR-COV19 has the prospect of being an effective single-dose vaccine without the mandatory booster injection, due to the benefits provided through the generation of self-transcription and replicative RNA of Arcturus (STARR) and its lipid-mediated LUNAR delivery vehicle.” Arce wrote in a note to investors. “With the dose of the recently introduced Phase 1/2 study, we continue to assign U.S. approval of LUNAR-COV19 at 2H21 through an Emergency Use Authorization (USA).”
Arce added that Arcturus is expected to supply Israel with a million doses of LUNARCOV19 and that the country is also entitled to purchase more doses of LUNAR-COV19 in two more tranches.
Overall, the inventory garnered a resounding purchase of five compared to the 1 retention of Wall Street analysts over the past 3 months, resulting in a strong Buy consensus. After this month’s uptick, the $66 average value target now indicates a prospective buildup of approximately 5.4% of existing levels.
Related news: Novartis’ candidate skin cancer drug does not focus on Gilead’s advanced trial says the FDA could expand the use of the reuser despite the data; PT Gilead swordsman sinks after general hours as FDA rejects arthritis claim
Tesla seeks to market a sensor to trip over an abandoned child in a hot car
Novartis skin cancer candidate drug fails to target advanced trial
Gilead states that the FDA can simply expand the use of remdesivir despite the data; Analyst hits PT
Deere earns 4% in its profit outlook by 2020